Serial Dilution Technique | For Microbiological & Chemical Analysis | Method, Example & Calculation
Summary
TLDRThis video provides an in-depth guide to the serial dilution technique, a crucial method in microbiological and chemical analysis. The process is broken down into three parts: preparing diluted samples, testing them microbiologically and chemically, and calculating the results. Viewers are shown how to perform each dilution step and how to analyze and calculate the concentration of analytes or microorganisms in the original sample. The video explains the dilution factors, testing procedures, and result calculations in a clear, step-by-step manner, making it an informative resource for those working in laboratory settings.
Takeaways
- 😀 The serial dilution technique is essential for accurate sample preparation in microbiological and chemical analysis.
- 😀 The video is divided into three main parts: performing the dilution, testing the samples, and calculating the results.
- 😀 Accurate dilution requires proper equipment: diluent, Falcon tube, vortex mixer, and micro-pipette.
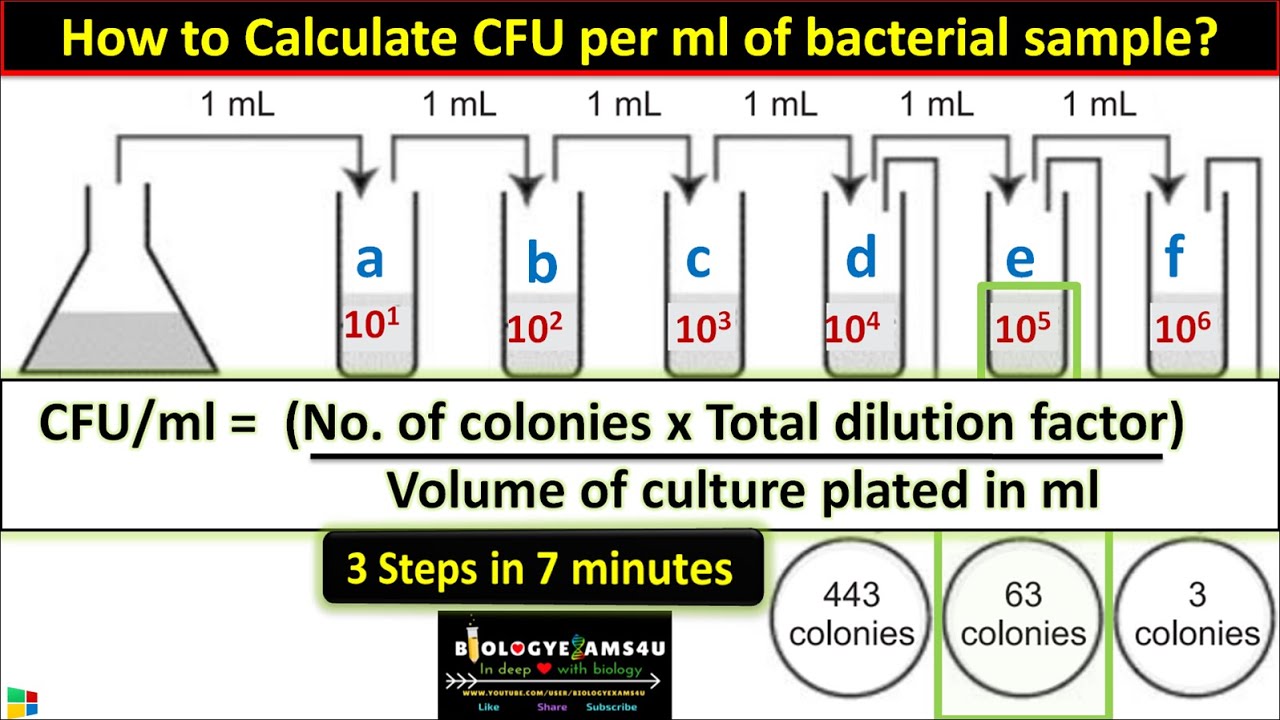
- 😀 The dilution process involves transferring a fixed volume of the sample into a diluent to decrease the concentration systematically.
- 😀 The first dilution uses 10 mL of the sample with 90 mL of diluent, yielding a dilution factor of 10.
- 😀 Subsequent dilutions (2-5) involve transferring 1 mL from the previous dilution into a new tube with 9 mL diluent, resulting in increasing dilution factors (100, 1000, etc.).
- 😀 Vortexing each dilution is crucial to ensure a homogeneous mixture and accurate results.
- 😀 Microbiological analysis involves spreading the diluted samples on agar plates to count colony-forming units (CFU).
- 😀 Chemical analysis involves calculating the concentration of analytes in the diluted samples.
- 😀 Calculation of final results is based on multiplying the diluted result by the dilution factor, such as 12 CFU at dilution 3 gives 721,000 CFU for the original sample.
- 😀 Uncountable colonies in dilutions 1 and 2 indicate high bacterial concentration, and specific CFU counts are obtained from dilutions 3-5 for more accurate analysis.
Q & A
What is the purpose of the serial dilution technique in microbiological and chemical analysis?
-The serial dilution technique is used to prepare a sample by diluting it multiple times to reduce the concentration of analytes. This allows for more accurate testing of microorganisms or chemicals in the original sample, which would otherwise be too concentrated to analyze effectively.
What are the key equipment items required for performing serial dilution?
-The key equipment required for serial dilution includes a Falcon tube, vortex mixer, micropipette with tips, and an appropriate diluent.
Why is vortexing important during the dilution process?
-Vortexing is essential to ensure that each dilution is homogeneous. Proper mixing ensures accurate and consistent test results by evenly distributing the analyte across the sample.
What is the dilution factor of the first dilution (dilution 1)?
-The dilution factor of the first dilution is 10, or 10^-1. This is calculated by dividing the final volume (100 mL) by the sample volume (10 mL).
How does the dilution factor change with each subsequent dilution?
-The dilution factor increases by a factor of 10 with each subsequent dilution. For example, dilution 1 has a factor of 10^-1, dilution 2 has a factor of 10^-2, and so on up to dilution 5, which has a factor of 10^-5.
What happens if the colony count in dilution 1 or 2 is too high to count?
-If the colony count in dilution 1 or 2 is too high to count, it means the colony density is excessive. In such cases, accurate results can only be derived from the more dilute samples (such as dilution 3, 4, or 5), where the colony count is within a manageable range.
How is the final result of the original sample calculated from a dilution?
-The final result of the original sample is calculated by multiplying the diluted result by the dilution factor for the respective dilution. For example, if 12 colony-forming units (CFUs) are observed in dilution 1, the final result is 12 multiplied by the dilution factor (10^-1).
What is the calculation process for microbiological test results?
-In microbiological tests, the number of colony-forming units (CFUs) is counted in the diluted samples. The CFU count is then multiplied by the dilution factor of that sample to calculate the final result for the original sample.
How do you calculate the chemical concentration results in serial dilution?
-To calculate chemical concentration results, the concentration observed in the diluted sample (e.g., PPM) is multiplied by the dilution factor. This gives the final concentration of the chemical in the original sample.
What are some potential applications of serial dilution in scientific research?
-Serial dilution is used in various fields such as microbiology, environmental science, pharmaceuticals, and chemistry to quantify microbial growth, chemical concentrations, or to determine the presence of specific substances in a sample.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
5.0 / 5 (0 votes)