Pharmacokinetics | Drug Excretion
Summary
TLDRThe video discusses key concepts in drug absorption, reabsorption, and excretion, focusing on how urine pH can be manipulated to enhance drug elimination in cases of overdose. By increasing proton concentration to acidify the urine, drugs can be trapped in their charged form, aiding in excretion. Conversely, in cases of weak acid overdoses, bicarbonate is used to decrease proton concentration, promoting drug absorption. The video also touches on pharmacokinetic concepts like clearance, half-life, and enzyme kinetics, offering practical insights for managing drug overdoses effectively.
Takeaways
- 😀 The process of excretion in the body involves complex mechanisms, including the manipulation of urine pH to control the absorption and excretion of drugs or ions.
- 😀 Nonpolar molecules are easier to absorb by the body, while polar molecules (like B-H+) are typically excreted in the urine.
- 😀 To enhance the absorption of nonpolar molecules, the body can shift the chemical equilibrium to favor the nonpolar form by increasing proton concentration.
- 😀 Increasing proton concentration in the kidneys can be achieved by acidifying the urine, which helps trap polar forms and prevent reabsorption.
- 😀 Ammonium chloride is a drug used to acidify the urine, thereby increasing the proton concentration and enhancing excretion of certain molecules.
- 😀 For weak acid overdoses, bicarbonate is used to decrease proton concentration, which reduces the ability of weak acids to be reabsorbed in the kidneys, promoting their excretion.
- 😀 The principle of urine pH manipulation is particularly useful in overdose scenarios to help remove toxic substances more efficiently from the body.
- 😀 The reabsorption process can be modified by changing the acidity or alkalinity of urine, which directly impacts the retention or excretion of substances in the body.
- 😀 Understanding how to manipulate the kidney’s reabsorption processes is critical in managing the excretion of drugs and other substances in clinical settings.
- 😀 The concept of clearance and half-life, as well as enzyme kinetics, will be discussed next to understand how drugs are cleared from the body more comprehensively.
Q & A
What role do weak acids and weak bases play in drug absorption and excretion?
-Weak acids and weak bases dissociate into charged or polar forms in the body. The charged forms are more difficult to reabsorb in the kidneys, while the nonpolar forms are easier to absorb. The ability to influence the dissociation helps control the excretion or absorption of these substances.
How does urine pH affect the reabsorption of weak acids and bases?
-Urine pH influences the dissociation of weak acids and bases. Acidifying the urine increases proton concentration, shifting the equilibrium toward the nonpolar, reabsorbable form of a weak base. Conversely, alkalizing the urine helps trap charged molecules, facilitating their excretion.
Why is ammonium chloride given to patients in overdose cases?
-Ammonium chloride is given to acidify the urine, increasing proton concentration. This helps shift the equilibrium to the nonpolar form of weak bases, making it more difficult for the kidneys to reabsorb the substance, thereby promoting its excretion.
What is the purpose of bicarbonate in cases of weak acid overdoses?
-Bicarbonate is used to alkalize the urine, decreasing proton concentration. This shifts the equilibrium away from the nonpolar form, helping to trap weak acids in their charged form and promoting their excretion.
What happens if a molecule stays in its polar form in the kidneys?
-If a molecule stays in its polar form, it is more likely to be excreted rather than reabsorbed, as charged molecules are harder for the kidneys to reabsorb.
How does increasing proton concentration in urine impact weak bases?
-Increasing proton concentration in urine shifts the equilibrium of weak bases toward their nonpolar form, making them more likely to be absorbed in the body instead of being excreted.
Why can't we decrease proton concentration directly to control drug absorption?
-Decreasing proton concentration directly is not always effective because the concentration of protons is already naturally present. Instead, increasing the concentration of protons or using other methods like ammonium chloride or bicarbonate is a more practical approach.
What is the overall goal of modifying the pH of urine in drug excretion?
-The goal of modifying the pH of urine is to enhance the excretion of certain drugs or toxins by either trapping them in their polar (charged) form or promoting their absorption by shifting them to their nonpolar form.
How can changing the urine's acidity or alkalinity help in managing overdoses?
-By altering the urine’s acidity or alkalinity, we can control the ionization of drugs or toxins. Acidifying urine helps excrete weak bases, while alkalizing it helps excrete weak acids, aiding in the elimination of overdosed substances.
What is the connection between urine pH manipulation and enzyme kinetics in drug clearance?
-Manipulating urine pH can influence drug clearance by affecting the ionization and reabsorption rates of molecules. While enzyme kinetics typically involve the metabolism of substances, altering urine pH can directly affect how substances are cleared from the body, complementing the process.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Drug Ionization

Pharmacokinetics: Drug absorption and distribution

Interaksi Obat dengan Makanan: Konsep Dasar
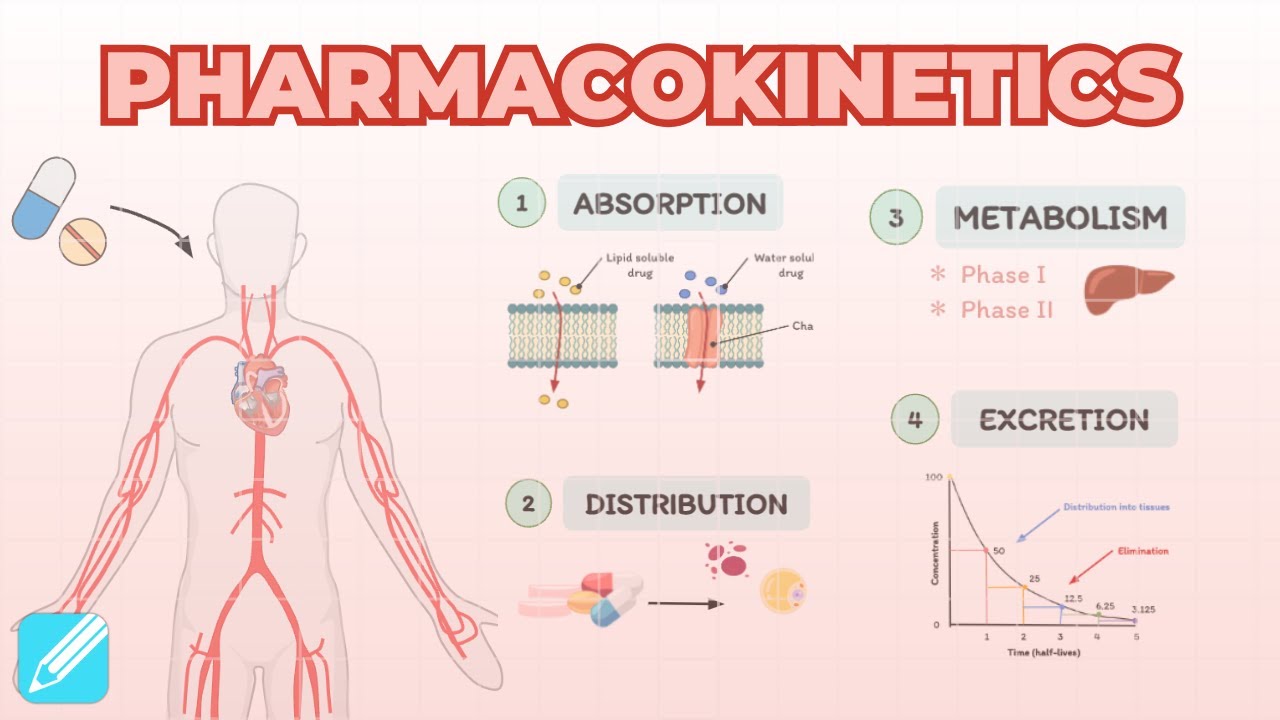
Pharmacokinetics: Absorption, Distribution, Metabolism & Excretion
Pharmacokinetics MADE EASY FOR BEGINNERS

Pharmacokinetics - What your body does to the med - Quick Review - Pharmacology Series
5.0 / 5 (0 votes)