4.3d Measuring Pressure: Manometer 'U Tubes' and Barometer Pipes | AS | Cambridge A Level Physics
Summary
TLDRThis video explains how to measure gas pressure using three primary devices: the manometer, barometer, and pressure gauge. It covers key fluid pressure principles, such as the relationship between liquid height and pressure, and the impact of atmospheric pressure. The manometer uses a liquid column to measure pressure differences, while the barometer measures atmospheric pressure via mercury in a tube. The pressure gauge operates by measuring gas pressure through a mechanical lever system. The video also discusses important safety considerations when handling mercury and provides formulas for calculating pressure using liquid density and height.
Takeaways
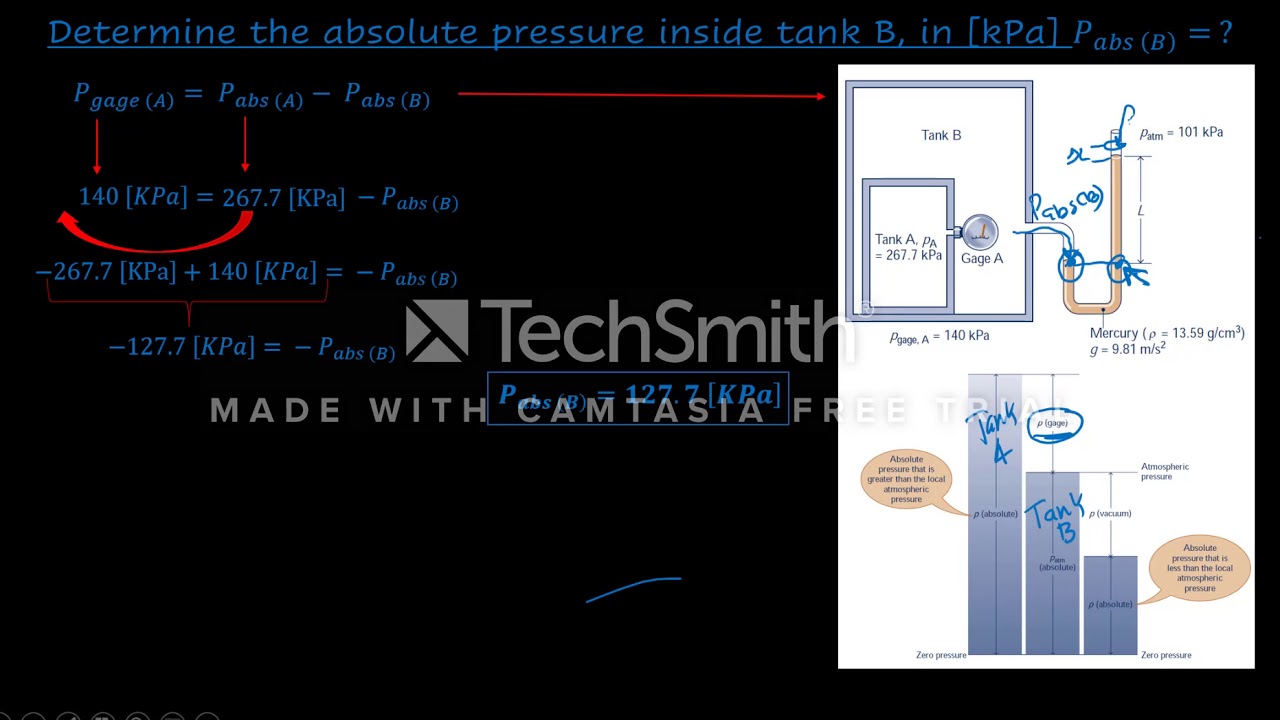
- 😀 Fluid pressure is defined as the force exerted per unit area by a fluid and can be calculated using the equation p = density × height × gravitational constant.
- 😀 A manometer is a device used to measure gas pressure, typically consisting of a U-shaped tube filled with a liquid like mercury.
- 😀 Atmospheric pressure acts on both open ends of the manometer, affecting the liquid levels and indicating the relative pressures.
- 😀 When the pressure inside the gas column increases, it causes a height difference in the liquid column, which is used to measure gas pressure.
- 😀 The principle of 'same height, same pressure' ensures that at equal heights in a fluid column, the pressure is the same.
- 😀 A barometer, similar to a manometer, is used to measure atmospheric pressure by using a column of mercury and observing the height of the mercury column.
- 😀 Mercury is used in barometers because of its high density, which allows for a more compact measurement compared to using water.
- 😀 At sea level, atmospheric pressure is approximately 760 mm of mercury, which can be measured with a barometer.
- 😀 If a barometer is tilted, the height of the liquid column remains the same because fluid pressure is equal in all directions.
- 😀 A pressure gauge, or Bourdon gauge, is a device used to measure the pressure of gases in applications like car tires. It operates by rotating a pointer using a lever system.
Q & A
What is the basic formula for fluid pressure mentioned in the video?
-Fluid pressure is calculated as the product of density (ρ), height (h) of the fluid column, and the gravitational constant (g). The formula is: P = ρ × h × g.
What role does atmospheric pressure play in the manometer demonstration?
-Atmospheric pressure acts on both open ends of the manometer. On one side, atmospheric pressure presses down on the liquid, causing equilibrium. When gas pressure is introduced on the other side, it causes the liquid levels to change.
Why is mercury often used in manometers and barometers?
-Mercury is used because of its high density, which allows for a more manageable column height compared to less dense liquids like water. It also has low volatility and remains liquid at a wide range of temperatures.
How does the manometer indicate gas pressure?
-The manometer indicates gas pressure by measuring the difference in liquid column heights when gas is compressed into one arm of the tube. The pressure difference causes the liquid to rise in one arm and fall in the other.
What does the barometer measure, and how does it work?
-A barometer measures atmospheric pressure. It works by using a column of mercury inside a tube. Atmospheric pressure pushes mercury up the tube, and the height of the mercury column reflects the pressure.
Why is it important to clean up mercury if it spills?
-Mercury is highly toxic, and if it comes into contact with skin or is inhaled, it can cause poisoning, leading to symptoms like delirium. Proper cleanup is essential to avoid exposure to this harmful substance.
What happens to the height of the mercury column in a barometer at higher altitudes?
-At higher altitudes, atmospheric pressure decreases, which results in a shorter mercury column in the barometer. This is why the height of the mercury column is lower than the standard sea-level height of 760 mm.
What does the pressure gauge measure, and how does it function?
-A pressure gauge, or Bourdon gauge, measures gas pressure. It uses a coiled tube that bends as gas pressure is applied. This bending moves a needle or pointer to indicate the pressure level.
How does the principle of 'same height, same pressure' apply to the devices discussed?
-The principle 'same height, same pressure' means that when the liquid columns in a manometer or barometer are at the same height, the pressures at those points are equal. This allows for accurate measurements of gas or atmospheric pressure.
Why would using water instead of mercury in a barometer be impractical?
-Water has a much lower density than mercury, so a barometer filled with water would require a very tall column (about 10 meters) to measure the same atmospheric pressure, which is impractical for standard use.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade Now5.0 / 5 (0 votes)