Atomic Structure: The Basics
Summary
TLDRIn this video, the host introduces basic concepts of atomic structure, highlighting the contributions of scientists like WH Fox Talbot in discovering bright line spectra, a key method for identifying elements. The discussion covers fundamental atomic properties such as protons, neutrons, electrons, and atomic number. It also touches on topics like atomic mass, the role of subscripts and coefficients in chemical equations, and the importance of the periodic table. With a mix of historical context and foundational chemistry, the video serves as an engaging overview of atomic theory.
Takeaways
- 😀 WH Fox Talbot is credited with discovering that each element produces a unique bright-line spectrum, which can be used for identification.
- 😀 A bright-line spectrum occurs when an element is heated, and its light is analyzed through a spectroscope. Each element has its own characteristic spectrum.
- 😀 The bright-line spectra were crucial for understanding atomic structure and identifying elements in the early days of chemistry.
- 😀 Modern atomic theory and the work of chemists like Swan helped refine the understanding of bright-line spectra, emphasizing the need for ultra-pure samples.
- 😀 Element names often reflect either their properties (like indium, named for the indigo color of its spectrum) or places (like polonium, named after Poland).
- 😀 Subscripts in chemical formulas indicate the number of atoms of an element in a compound, while coefficients in front of compounds indicate the quantity of molecules.
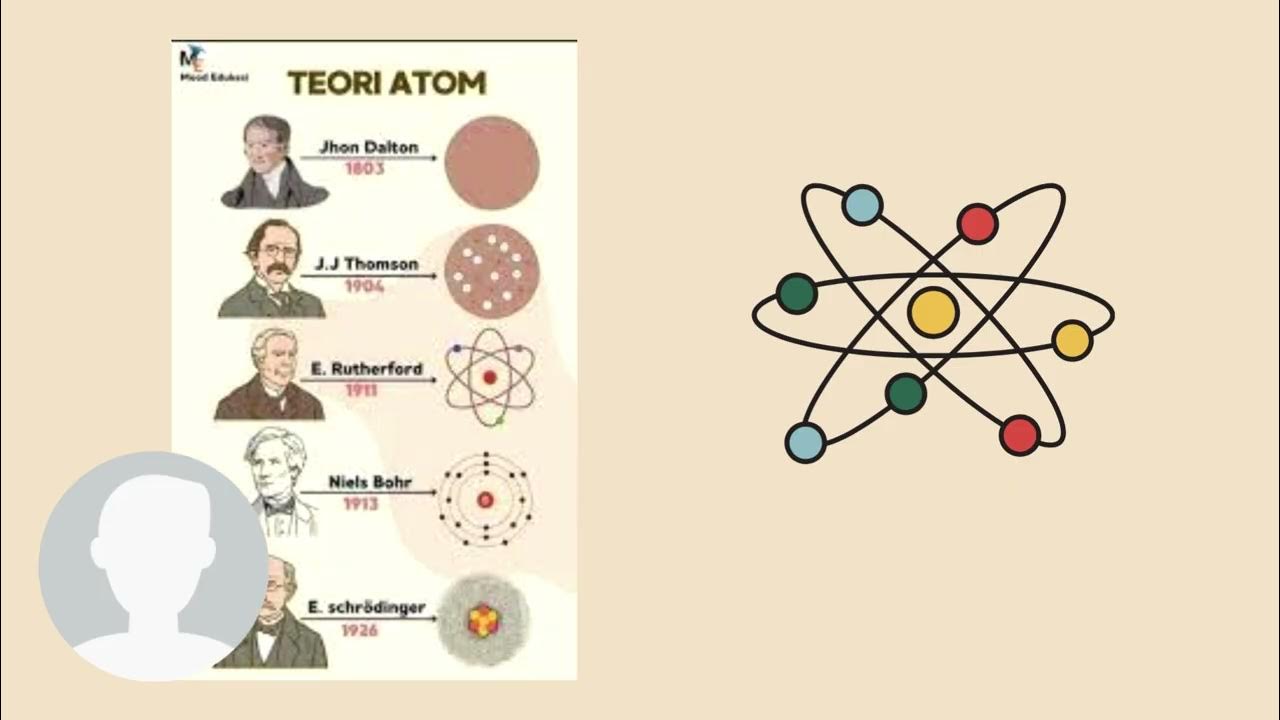
- 😀 Atomic mass (also called mass number) is the sum of protons and neutrons in an atom, while the atomic number refers to the number of protons.
- 😀 Electrons are much lighter than protons and neutrons, and most of an atom's mass is concentrated in the nucleus, which is much smaller than the whole atom.
- 😀 Atoms are incredibly small, with a typical size range of 1 to 5 angstroms (10^-10 meters), but they contain nearly all their mass in a tiny nucleus.
- 😀 The atomic number is critical for determining the identity of an element. Changing the number of protons changes the element itself.
- 😀 In a neutral atom, the number of protons equals the number of electrons. If they are not equal, the atom becomes an ion.
Q & A
Who was W.H. Fox Talbot, and what is his contribution to atomic theory?
-W.H. Fox Talbot was a chemist and photographer known for discovering that each element emits a unique bright line spectrum when heated. This discovery played a pivotal role in the development of spectroscopy, a technique used to identify elements based on their characteristic light emissions.
What is a bright line spectrum, and why is it important in atomic theory?
-A bright line spectrum is the pattern of specific wavelengths of light emitted by an element when its atoms are excited. This spectrum is unique to each element, allowing scientists to identify elements based on the colors of light they emit, a crucial tool in early atomic theory.
What role did Swan play in improving spectroscopy?
-Thirty years after Fox Talbot's discovery, Swan contributed to improving spectroscopy by realizing that ultra-pure samples were needed to produce a clear, reliable bright line spectrum. This advancement helped refine the technique and make it more effective for identifying elements.
How does a spectroscope work?
-A spectroscope works by heating a sample of an element until it emits light. This light passes through a prism, which separates the light into its component colors. The resulting spectrum is unique to the element and can be analyzed to identify it.
What is the difference between a subscript and a coefficient in chemical formulas?
-A subscript in a chemical formula indicates the number of atoms of an element in a compound. A coefficient modifies the entire compound, affecting the number of molecules of the compound in a reaction. Subscripts are fixed for a given compound, while coefficients can be changed to balance chemical equations.
What is the atomic number, and why is it important?
-The atomic number is the number of protons in the nucleus of an atom. It is crucial because it determines the identity of the element. For example, every atom with 6 protons is carbon, and changing the number of protons would change the element entirely.
What is atomic mass, and how is it calculated?
-Atomic mass is the total mass of an atom, calculated by adding the number of protons and neutrons in the nucleus. It is commonly referred to as the mass number, and while the atomic mass may vary slightly due to isotopes, the mass number gives a close approximation.
What is the significance of atomic mass units (AMUs)?
-Atomic mass units (AMUs) are a standard unit of mass used to measure atoms and subatomic particles. One AMU is approximately the mass of a proton or neutron, making it a convenient unit for dealing with the tiny masses of atoms and particles.
What did Donna Angler achieve in 1989 in relation to atomic structure?
-In 1989, Donna Angler achieved a significant milestone by arranging atoms on an atomic scale, demonstrating the ability to manipulate and observe individual atoms. This achievement helped further our understanding of atomic structure and contributed to advancements in nanotechnology.
Why do atomic numbers have such a significant role in defining elements?
-Atomic numbers are crucial because they directly determine the number of protons in an atom's nucleus, which defines the chemical identity of the element. The atomic number is unique to each element, and even a small change in the number of protons would result in a completely different element.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade Now5.0 / 5 (0 votes)