Thermodynamic systems | Open System | Closed System | Isolated System | Basic Mechanical Engineering
Summary
TLDRThis video explores the fundamental concepts of thermodynamic systems, including open, closed, and isolated systems. It explains how energy and matter interact within each type of system, using practical examples like gas turbines, pressure cookers, and insulated thermoses. The video aims to provide a clear understanding of energy transfer, system boundaries, and their applications in real-world scenarios. By simplifying these complex concepts, the video helps viewers grasp essential thermodynamic principles and their significance in engineering and scientific fields.
Takeaways
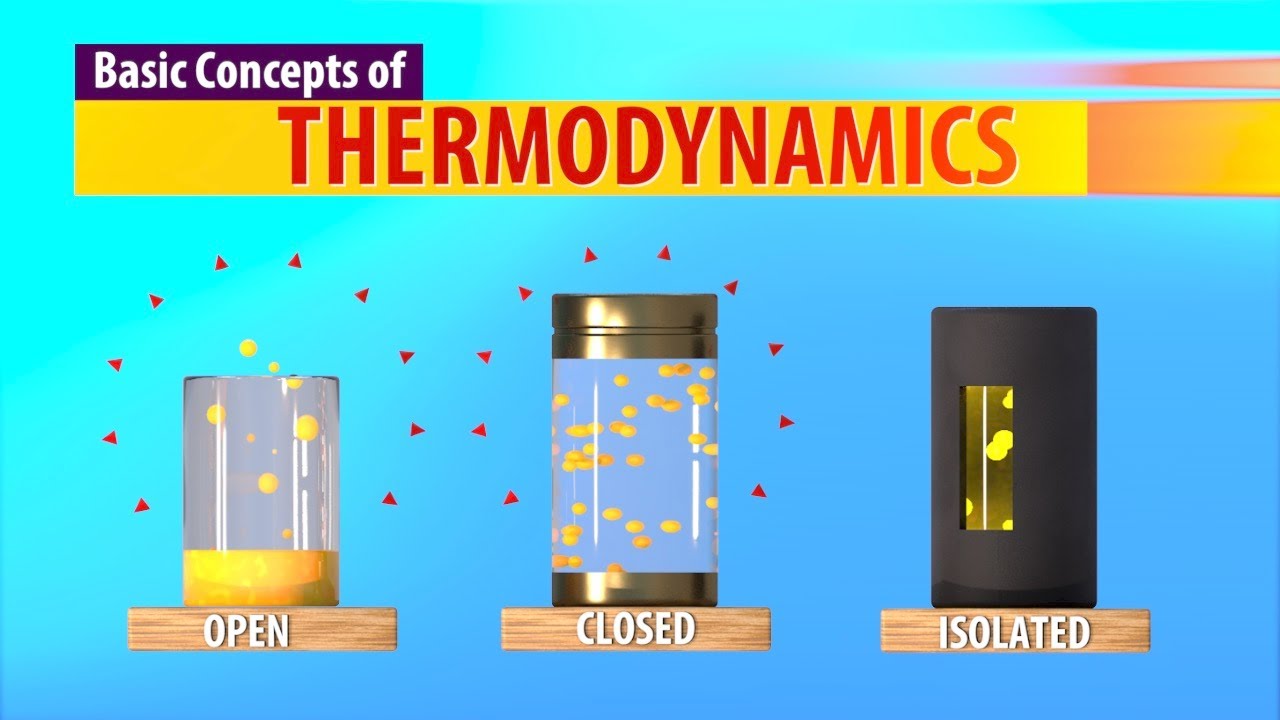
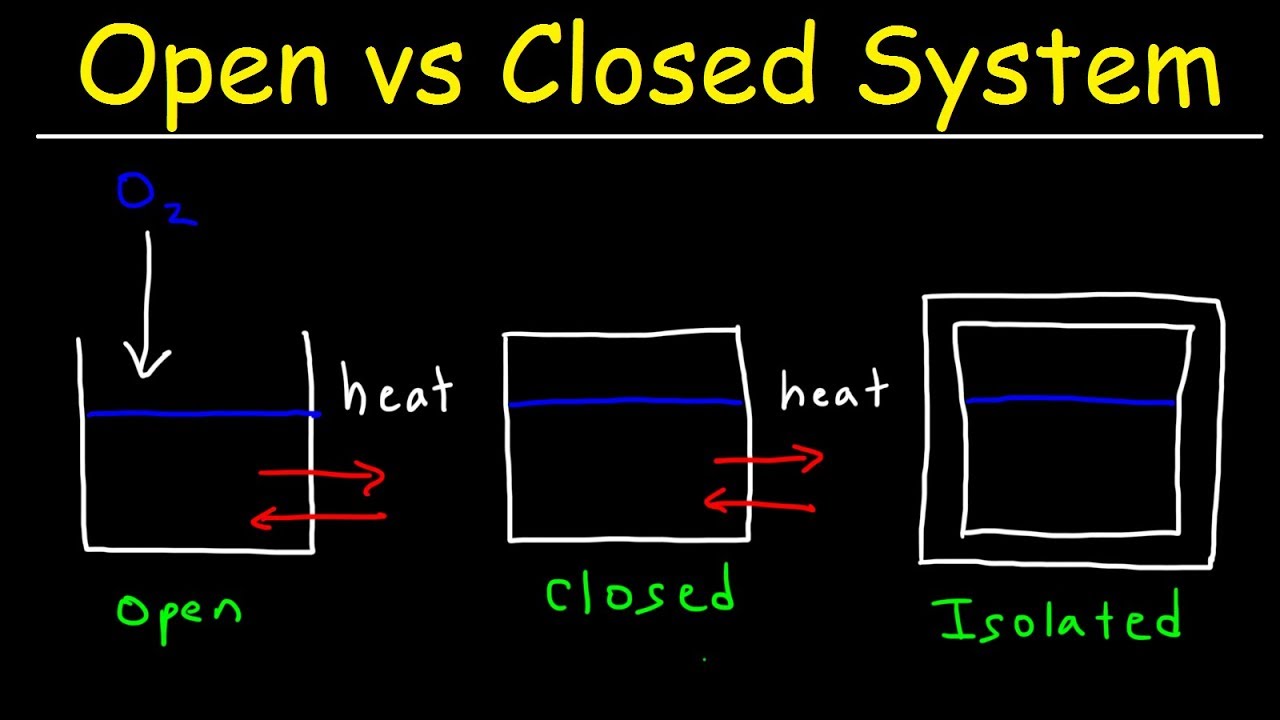
- 😀 Open systems allow the exchange of both matter and energy with their surroundings. Example: A boiling pot of water.
- 😀 Closed systems only exchange energy (heat or work) but not matter with their surroundings. Example: A sealed pressure cooker.
- 😀 Isolated systems do not exchange energy or matter with their surroundings. Example: A perfectly insulated thermos.
- 😀 Thermodynamic systems are classified based on how they interact with their surroundings: open, closed, and isolated systems.
- 😀 Energy transfer in thermodynamics occurs mainly in the form of heat and work, depending on the system's properties and boundaries.
- 😀 Heat transfer happens when temperature differences exist between the system and its surroundings. This occurs across the boundary.
- 😀 Work transfer happens when a system performs a physical action, like a gas pushing against a piston.
- 😀 The system's boundary can be real (like a container wall) or imaginary (in theoretical models).
- 😀 The thermodynamic system's state is defined by its properties like temperature, volume, and pressure.
- 😀 Understanding energy flow in thermodynamic systems helps in practical applications like engines, refrigerators, and power plants.
Q & A
What are the three types of thermodynamic systems mentioned in the script?
-The three types of thermodynamic systems are Open, Closed, and Isolated systems. An open system exchanges both matter and energy with its surroundings, a closed system exchanges energy but not matter, and an isolated system does not exchange either matter or energy.
Can you explain the concept of an open system in thermodynamics?
-An open system allows both matter and energy to be exchanged with its surroundings. For example, a gas turbine or an engine in a vehicle is an open system, as fuel and air enter the system, and exhaust gases exit.
What is an example of a closed system as per the script?
-A pressure cooker is an example of a closed system. It allows energy (in the form of heat) to be exchanged but keeps the matter (food and water) confined inside.
How does energy transfer in an isolated system?
-In an isolated system, ideally, no energy or matter is exchanged with the surroundings. A perfect example of this is a thermos bottle, which is insulated to prevent energy (heat) and matter (liquids) from transferring in or out.
What role do boundaries play in thermodynamic systems?
-Boundaries define the separation between a system and its surroundings. These boundaries control the flow of energy and matter. For instance, in a closed system like a pressure cooker, the boundary keeps the contents inside while allowing heat to escape.
How is energy transferred in an open system?
-In an open system, energy can be transferred through both heat and work. For example, in a gas turbine, heat is transferred to the surroundings, and mechanical work is done by the system as it powers machinery.
What is meant by 'heat transfer' in thermodynamic systems?
-Heat transfer refers to the movement of thermal energy from one body or system to another. In open and closed systems, heat can flow across the boundaries, impacting the system's energy state.
Can an isolated system ever transfer energy? If so, how?
-In theory, an isolated system does not transfer energy or matter. However, real-world systems may not be perfectly insulated, and slight energy transfer could occur through imperfect boundaries.
What is an example of energy transfer from the surroundings to a system?
-In a closed system, such as a pressure cooker, energy is transferred from the surrounding heat source to the food inside. The cooker absorbs heat, causing the contents to cook without allowing matter to escape.
How do the examples of thermodynamic systems in the script relate to everyday technology?
-Everyday technology such as refrigerators, engines, and thermos bottles all function as thermodynamic systems. Refrigerators are closed systems, car engines are open systems, and thermos bottles act as isolated systems, all demonstrating energy and matter exchange principles.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Termodinamika Kelas XI IPA

Basic Concepts of Thermodynamics [Year - 1]
Basic Concepts of Thermodynamics (Animation)

Termodinamika: Sistem terisolasi, tertutup, dan terbuka

Hukum Termodinamika, Bagian 1: Energi Dalam dan Hukum Pertama
Open System, Closed System and Isolated System - Thermodynamics & Physics
5.0 / 5 (0 votes)