Abnormal (misfolded) prions - Medical microbiology animations
Summary
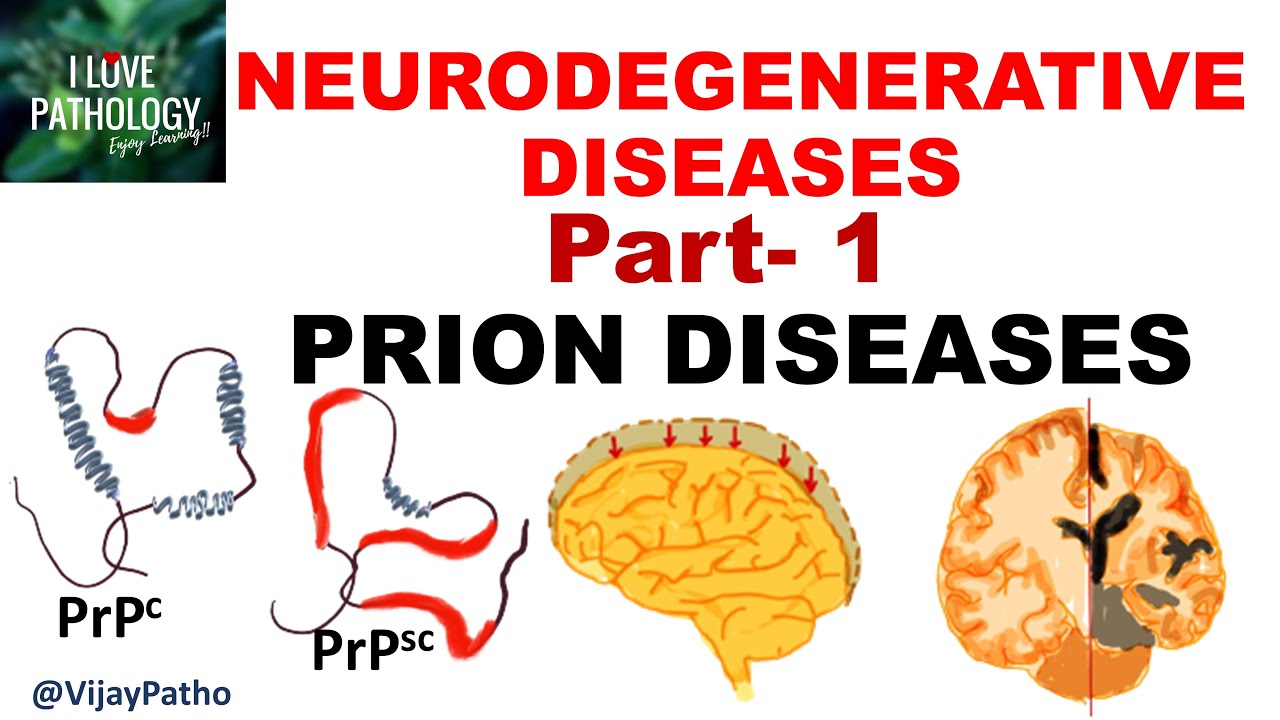
TLDRThe transcript discusses the role of prions in brain diseases like Creutzfeldt-Jakob disease and mad cow disease. It explains how the infectious agent is believed to be an altered prion protein (PrPSc) that induces normal prion proteins (PrPC) to misfold, thereby creating a cycle of protein misfolding and disease spread. Researchers suggest that PrPSc may also activate enzymes to modify PrPC structure. This highlights the complex mechanism of prion infection and its impact on neurological health.
Takeaways
- 😀 Prions are infectious agents associated with brain diseases like Creutzfeldt-Jakob disease and mad cow disease.
- 😀 A prion is a proteinaceous infectious particle, meaning it's made up of protein.
- 😀 The normal prion protein (PRP^C) is found on the surfaces of neurons in both vertebrates and invertebrates.
- 😀 The gene for normal prion protein (PRP^C) is present in most vertebrates and invertebrates.
- 😀 PRP^C can become misfolded and transform into an abnormal form, PRP^Sc (scrapie-associated).
- 😀 The misfolded prion protein (PRP^Sc) is believed to be the infectious agent that causes prion diseases.
- 😀 When PRP^Sc enters a healthy brain, it binds to normal PRP^C proteins and induces them to misfold.
- 😀 The misfolded PRP^C proteins (converted to PRP^Sc) can then cause a chain reaction, further spreading the abnormal conformation.
- 😀 This process leads to the brain damage seen in prion diseases.
- 😀 An alternative hypothesis suggests that PRP^Sc may activate enzymes that modify PRP^C's structure, contributing to the infection.
Q & A
What are prions, and how are they related to certain brain diseases?
-Prions are misfolded proteins that are associated with neurodegenerative diseases like Creutzfeldt-Jakob Disease in humans and Mad Cow Disease in animals. These diseases are believed to be caused by the abnormal folding of the prion protein.
What is the normal form of the prion protein called?
-The normal form of the prion protein is called PRP^C (prion protein cellular). It is bound to the surfaces of neurons and is found in most vertebrates and some invertebrates.
What happens when the prion protein changes from its normal form to the infectious form?
-When PRP^C is altered into the infectious form, known as PRP^SC (prion protein scrapie-associated), it becomes misfolded and adopts a different conformation that can propagate the disease.
How does PRP^SC propagate the prion disease in the brain?
-PRP^SC induces the normal PRP^C in healthy cells to fold abnormally into the infectious form. This misfolding can then continue to spread as more normal PRP^C proteins are converted into PRP^SC.
What is the role of PRP^SC in the transmission of prion diseases?
-PRP^SC acts as the infectious agent in prion diseases. It binds to normal PRP^C and induces it to adopt the misfolded, infectious conformation, thus spreading the disease.
Are there any alternative hypotheses about how prion diseases are caused?
-Yes, an alternative hypothesis suggests that PRP^SC may activate enzymes that modify the structure of PRP^C, leading to the formation of the infectious prion protein, though this idea is less widely accepted than the misfolding hypothesis.
What is the significance of the prion protein in vertebrates and invertebrates?
-The prion protein gene (PRP) is present in both vertebrates and invertebrates, indicating that the protein may have a normal, biological role in these organisms. However, when misfolded, it becomes an agent of disease.
How does the process of prion protein misfolding contribute to neurodegeneration?
-The misfolding of prion proteins leads to the formation of aggregates that accumulate in the brain, disrupting normal cell function and causing neurodegeneration, which can result in fatal diseases like Creutzfeldt-Jakob Disease.
Why is it believed that prions can spread without genetic material?
-Prions can spread because they are proteins that can self-replicate through misfolding, unlike most infectious agents, which require genetic material (DNA or RNA) to replicate. The abnormal prion protein acts as a template for other proteins to adopt its harmful structure.
Can prion diseases affect both humans and animals?
-Yes, prion diseases can affect both humans (such as Creutzfeldt-Jakob Disease) and animals (such as Mad Cow Disease in cattle and scrapie in sheep). The mechanism of misfolding and transmission of prions is similar across species.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade Now5.0 / 5 (0 votes)