Hipotesis Avogadro - Hukum Dasar kimia - kimia kelas 10
Summary
TLDRThis video explores Avogadro's Hypothesis, explaining how equal volumes of gases at the same temperature and pressure contain the same number of molecules. It discusses how volume ratios in chemical reactions correspond to stoichiometric coefficients and provides practical examples, such as the decomposition of ammonia and combustion of ethyne. The video also covers problem-solving techniques, demonstrating how to balance chemical equations and calculate unknown quantities, like the formula of a hydrocarbon. Overall, it highlights the importance of Avogadro's Hypothesis in understanding chemical reactions and stoichiometry.
Takeaways
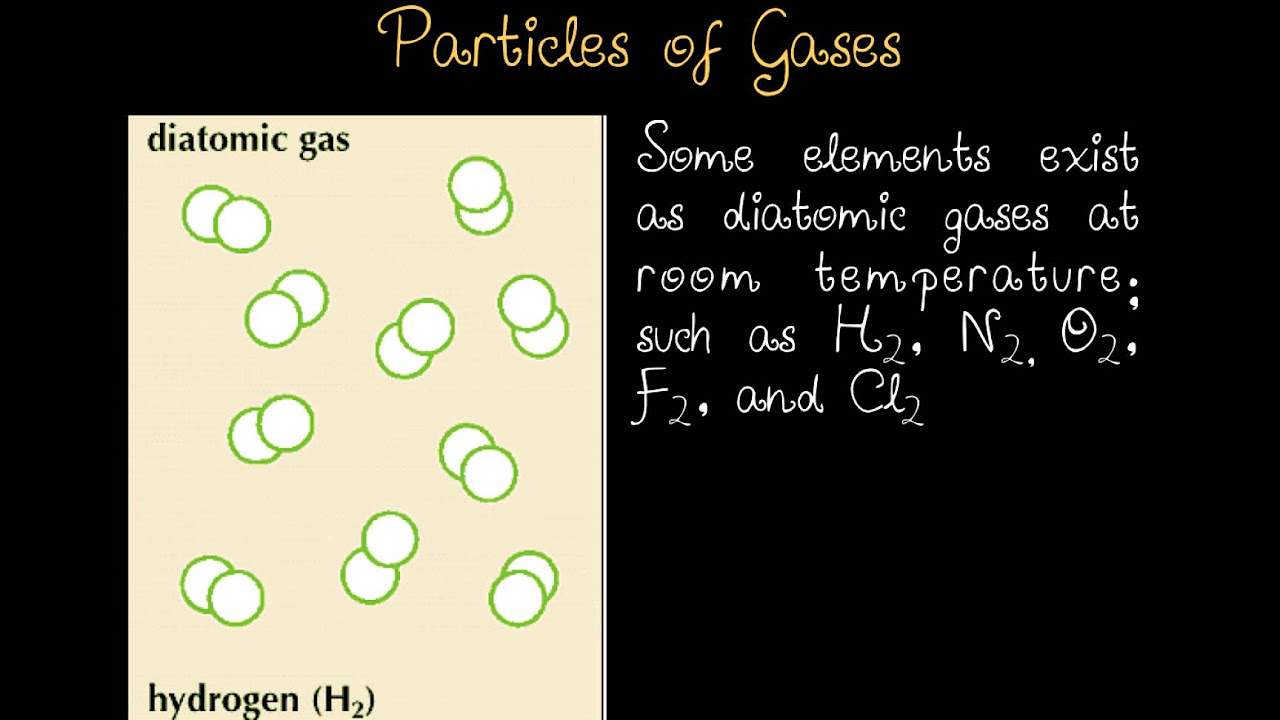
- 😀 Avogadro's Hypothesis states that, at constant temperature and pressure, equal volumes of gases contain an equal number of molecules or particles.
- 😀 The volume ratio of gases in a chemical reaction corresponds to the ratio of their respective coefficients in the balanced equation.
- 😀 For example, in the reaction 2 H2 + O2 → 2 H2O, the volume ratio of H2, O2, and H2O is 2:1:2, based on the coefficients.
- 😀 In the reaction H2 + Cl2 → 2 HCl, the volume ratio of H2, Cl2, and HCl is 1:1:2, reflecting the coefficients.
- 😀 Another example, N2 + 3 H2 → 2 NH3, shows the volume ratio of N2, H2, and NH3 as 1:3:2.
- 😀 When solving for the decomposition of NH3 into N2 and H2, the mole ratio is determined by balancing the reaction and applying Avogadro's Hypothesis.
- 😀 A balanced reaction like NH3 → N2 + 3 H2 suggests that if 2x moles of NH3 decompose, x moles of N2 and 3x moles of H2 are formed.
- 😀 In the combustion of acetylene (C2H2 + O2 → CO2 + H2O), the reaction must first be balanced before determining the volume ratios.
- 😀 The balanced reaction for acetylene combustion shows that the volume ratio of the reactants and products is 2:5:4:2, after adjusting the coefficients.
- 😀 For determining the empirical formula of a hydrocarbon from given volumes of reactants and products, Avogadro's Hypothesis is used to match the volumes with their coefficients in the balanced equation.
Q & A
What is Avogadro's hypothesis?
-Avogadro's hypothesis states that at the same temperature and pressure, equal volumes of different gases contain the same number of molecules or particles.
How is Avogadro's hypothesis applied in chemical reactions?
-Avogadro's hypothesis is used to relate the volume of gases in a chemical reaction to the number of molecules involved, as the volume ratios are proportional to the coefficients in a balanced chemical equation.
What is the relationship between volume ratios and molecular ratios in chemical reactions?
-The volume ratio of gases in a reaction is equivalent to the ratio of the molecules or moles of those gases, which is also reflected by the coefficients in the balanced chemical equation.
How do you calculate the volume ratio of gases in a balanced equation?
-To calculate the volume ratio, simply use the coefficients of the gases in the balanced equation. The volume ratio corresponds to the ratio of the coefficients of the gases involved.
In the reaction 2 H2 (g) + O2 (g) → 2 H2O (g), what is the volume ratio of H2, O2, and H2O?
-The volume ratio of H2 to O2 to H2O is 2:1:2, based on the coefficients in the balanced equation.
How do you balance a chemical equation like NH3 → N2 + H2?
-To balance this equation, start by equalizing the number of nitrogen (N) atoms on both sides. Then balance the hydrogen atoms by adjusting the coefficients to ensure the total number of atoms on each side is the same.
In the decomposition of NH3, if you start with 2x molecules of NH3, how much nitrogen and hydrogen are produced?
-According to the stoichiometry of the balanced reaction, if you start with 2x molecules of NH3, x molecules of nitrogen (N2) and 3x molecules of hydrogen (H2) are produced.
In the combustion reaction C2H2 + O2 → CO2 + H2O, how do you balance the equation?
-To balance the combustion reaction, adjust the coefficients for carbon (C), hydrogen (H), and oxygen (O) so that the number of atoms of each element is the same on both sides. After balancing, the coefficients should be 2:1:2:1.
What is the process for finding the formula of a hydrocarbon from a chemical reaction?
-To find the formula of a hydrocarbon, use the volume ratios from the balanced reaction to determine the number of carbon (C) and hydrogen (H) atoms, based on the given data for the reactants and products.
Given that 0.5 L of a hydrocarbon (CxHy) reacts with 1.75 L of oxygen to produce 1.5 L of water vapor, how do you determine the formula of the hydrocarbon?
-By using the volume ratios, we can set up a system of equations based on the balanced reaction. After balancing and comparing the volumes of the reactants and products, we find that the hydrocarbon has the formula C2H6.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade Now5.0 / 5 (0 votes)