💧 Cambios Físicos y Químicos 🔥 [Fácil y Rápido] | QUÍMICA |
Summary
TLDRThis video explains the differences between physical and chemical changes in nature. Physical changes involve alterations in the state or appearance of matter, such as cutting, dissolving, or changing states (solid, liquid, gas) without altering its internal structure. Chemical changes, or reactions, involve the formation of new substances with different characteristics, such as combustion, rusting, or digestion. The video also highlights key indicators of chemical changes, like color change, heat emission, or the formation of a precipitate. The video concludes with a thank-you message and a call to like, comment, and subscribe.
Takeaways
- 😀 Physical changes refer to alterations where the structure of matter doesn't change, and the material retains its original properties.
- 😀 An example of a physical change is the transformation of water between solid, liquid, and gas states without changing its fundamental properties.
- 😀 Other examples of physical changes include cutting a piece of paper, breaking glass, stretching a rubber band, or dissolving sugar in water.
- 😀 A chemical change, or chemical reaction, involves a change in the internal structure of matter, leading to new properties and characteristics.
- 😀 Burning a piece of paper and turning it into ash is an example of a chemical change, as the properties of the ash differ from those of the paper.
- 😀 A sign of a chemical change includes the formation of a precipitate (a solid forming when two liquids are mixed).
- 😀 Changes in color, emission of light or heat, and the formation of a permanent substance are also indicators of chemical reactions.
- 😀 Examples of chemical reactions include combustion (burning fuel), oxidation (rusting metal), and fermentation (e.g., in beer, wine, or bread).
- 😀 Other chemical changes include food digestion, fireworks, and the process of photosynthesis in plants.
- 😀 The video concludes by encouraging viewers to like, comment, share, and subscribe to the channel for more educational content.
Q & A
What are physical changes in nature?
-Physical changes are changes in which the structure of the matter does not alter. The properties and characteristics remain the same, such as the points of fusion and boiling of water, regardless of its state (solid, liquid, or gas).
Can you give examples of physical changes?
-Examples of physical changes include cutting a piece of paper into smaller pieces, converting wood into sawdust, breaking glass, stretching a rubber band, crumpling paper, melting snow, and stretching clay.
What are chemical changes?
-Chemical changes, also known as chemical reactions, involve a change in the internal structure of the matter, leading to the creation of new properties that were not present before the change.
How can you tell if a chemical change is happening?
-Signs of a chemical change include the formation of a solid (precipitation), color changes, emission of light or heat, or the formation of a permanent substance.
What is an example of a chemical change involving combustion?
-Combustion examples include the burning of gasoline, oil, a candle, or a forest fire. These processes involve a chemical reaction where new substances are created.
Can oxidation be considered a chemical change?
-Yes, oxidation is a chemical change. It occurs when materials like metal nails or an apple undergo oxidation or rusting, forming new substances that are different from the original.
What is the role of photosynthesis in chemical changes?
-Photosynthesis in plants is a chemical change. It involves the conversion of carbon dioxide and water into glucose and oxygen, driven by sunlight, which creates new substances and energy.
How is digestion a chemical change?
-Digestion is a chemical change because it involves the breakdown of food into smaller molecules, which requires enzymes and chemical reactions to transform the food into absorbable nutrients.
What are some examples of fermentation being a chemical change?
-Fermentation is a chemical change used in processes like brewing beer, making wine, or producing yogurt. During fermentation, microorganisms break down sugars into alcohol or acids, creating new substances.
Is freezing water a physical or chemical change?
-Freezing water is a physical change. While water changes from liquid to solid, its chemical structure (H2O) remains unchanged, and its properties such as the melting point stay the same.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

PASHU PARICHAR GENERAL SCIENCE MCQ CLASS | भौतिक एवं रासायनिक परिवर्तन | Most Questions By DBM SIR

Perubahan Materi / KIMIA kelas 10 kurikulum merdeka , perubahan fisika dan perubahan kimia
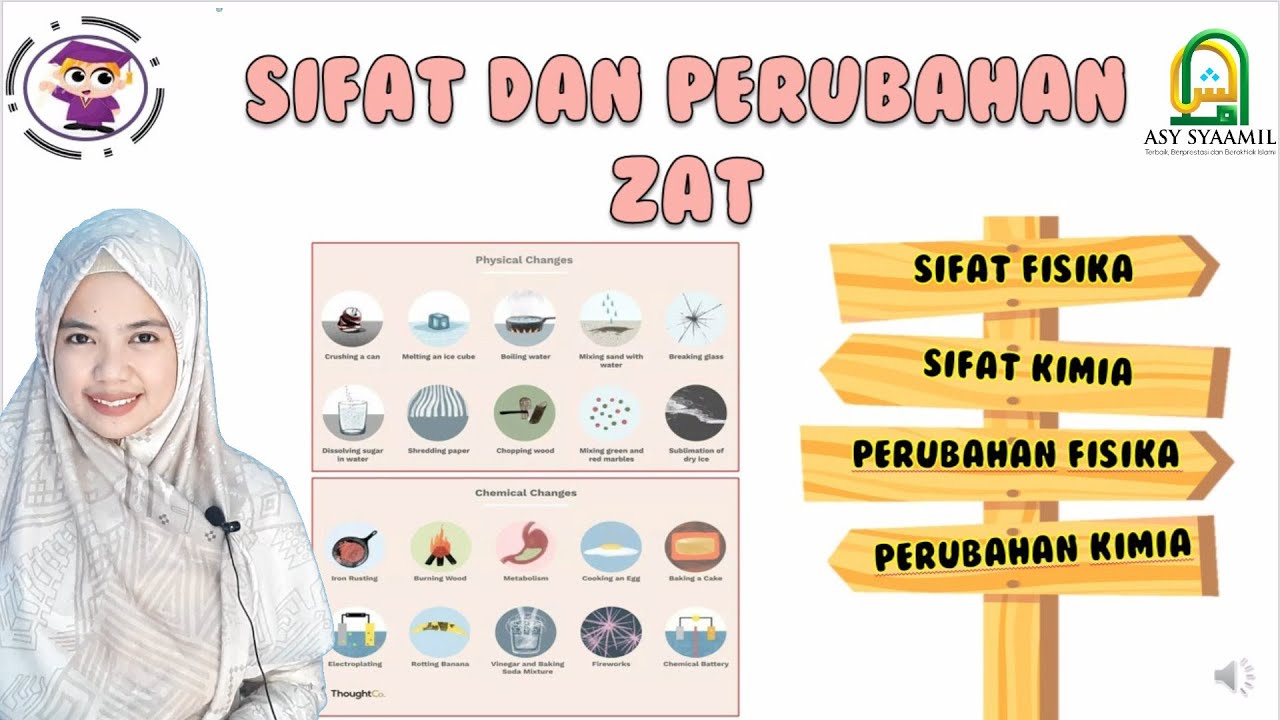
SIFAT DAN PERUBAHAN ZAT | SIFAT FISIKA DAN KIMIA | PERUBAHAN FISIKA DAN KIMIA

10th Science 1 | Chapter 3 | Chemical Reactions & Equations | Lecture 1 | Maharashtra Board |

Is Matter around us Pure in 20 Minutes🔥| Class 9th | Rapid Revision | Prashant Kirad

Physical and Chemical Properties and Changes
5.0 / 5 (0 votes)