Hybridation des orbitales atomiques (2) - sp2
Summary
TLDRIn this video, the speaker explores various types of hybridization beyond SP3, using the example of AlCl3 to illustrate how the central atom's electronic configuration informs its bonding behavior. By analyzing aluminum's valence electrons and the need for three equivalent energy orbitals, the video demonstrates the formation of SP2 hybridization. The discussion then transitions to C2H4, where carbon hybridizes in different ways depending on its bonding requirements, further emphasizing that atoms can hybridize in multiple configurations. A summary table is promised to simplify the identification of hybridization types in different molecules.
Takeaways
- 😀 Hybridization is a method to explain bonding by mixing atomic orbitals to form new hybrid orbitals.
- 😀 The most common types of hybridization are sp, sp2, and sp3, each associated with different bonding scenarios.
- 😀 In AlCl3, aluminum undergoes sp2 hybridization, forming three equivalent orbitals for bonding.
- 😀 The electronic configuration of aluminum is [Ne]3s²3p¹, leading to three valence electrons available for bonding.
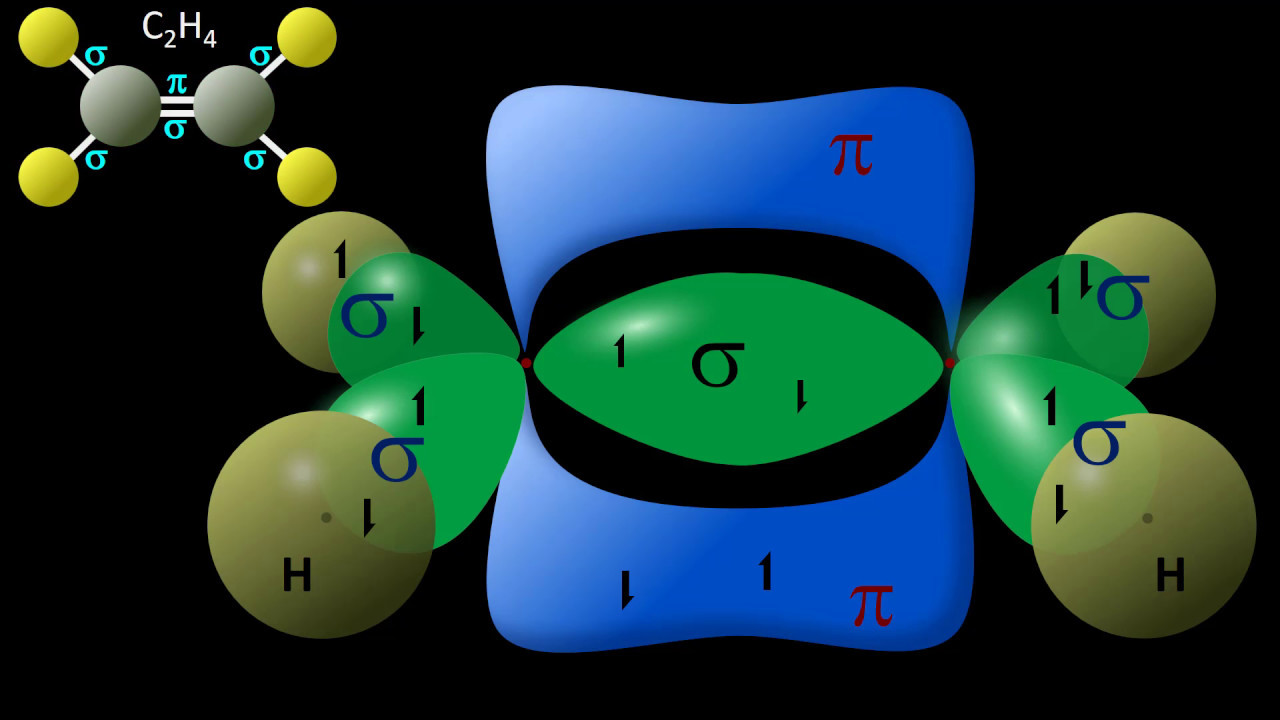
- 😀 Ethylene (C2H4) features carbon atoms that undergo sp2 hybridization due to the formation of a double bond.
- 😀 Each carbon in C2H4 has an electronic configuration of [He]2s²2p², which contributes to its hybridization.
- 😀 The hybridization process retains the number of orbitals; sp2 hybridization involves one s and two p orbitals.
- 😀 To determine hybridization, count the number of bonds an atom forms: four bonds indicate sp3, three bonds sp2, and two bonds sp.
- 😀 The concept of hybridization is essential for predicting molecular geometry and reactivity.
- 😀 Different atoms can hybridize in various ways depending on their molecular context, impacting the overall structure.
Q & A
What is the main focus of the video?
-The video focuses on different types of hybridization for central atoms in molecules, particularly aluminum in AlCl3 and carbon in C2H4.
How many valence electrons does aluminum have?
-Aluminum has three valence electrons, with an electronic configuration of 3s² 3p¹.
What type of hybridization does aluminum undergo in AlCl3?
-In AlCl3, aluminum undergoes SP2 hybridization to form three equivalent bonds.
What is the electronic configuration of carbon?
-Carbon has four valence electrons, with an electronic configuration of 1s² 2s² 2p².
What hybridization occurs for carbon in C2H4?
-In C2H4, carbon undergoes SP2 hybridization to accommodate a double bond and bonds with hydrogen.
What is the significance of hybridization in molecular bonding?
-Hybridization allows atoms to form bonds of equal energy and orientation, which is essential for creating stable molecular structures.
How does the number of bonds influence hybridization?
-The number of bonds formed by an atom helps determine its hybridization type: SP3 for four bonds, SP2 for three, and SP for two.
Can the same atom have different hybridizations in different molecules?
-Yes, an atom can have different hybridizations depending on the molecular context; for example, carbon can hybridize as SP3 in CH4 and as SP2 in C2H4.
What does the term 'hybrid orbital' refer to?
-A hybrid orbital is a new orbital formed by the combination of standard atomic orbitals, allowing for the formation of bonds with specific orientations.
What happens to unused orbitals during hybridization?
-Unused orbitals remain available at a higher energy level and do not participate in bonding, as seen in the cases of aluminum and carbon.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Hybridation des orbitales atomiques (1) - Intro & sp3

Hybridization of Atomic Orbitals - Sigma & Pi Bonds - Sp Sp2 Sp3

SE1x_2022_Week_2_3_3_Band_Gap_III-video
Hybrid Orbitals explained - Valence Bond Theory | Orbital Hybridization sp3 sp2 sp

VSEPR Theory and Molecular Geometry
BTEC Applied Science - Unit 5 Chemistry - sigma and pi bonding hybridisation
5.0 / 5 (0 votes)