Understand statistics in Cochrane systematic reviews
Summary
TLDRThis module introduces dietitians to the importance of peer-reviewing Cochrane systematic reviews, highlighting the focus on randomized control trials and the statistical methods involved. It covers key concepts such as dichotomous and continuous outcomes, meta-analysis, relative risks, odds ratios, and mean differences. The training emphasizes interpreting these statistics in a clinical context, encouraging dietitians to provide meaningful feedback based on their expertise. The module also outlines the significance of understanding confidence intervals and the relevance of clinical significance over mere statistical significance in health research.
Takeaways
- 📚 Overview of the project funded by the Canadian Institutes of Health Research to enhance dietitians' involvement in Cochrane systematic reviews.
- 👥 Acknowledgment of the support from various dietitians and review groups across Canada.
- 🔍 The series includes three modules focused on peer-reviewing Cochrane systematic reviews.
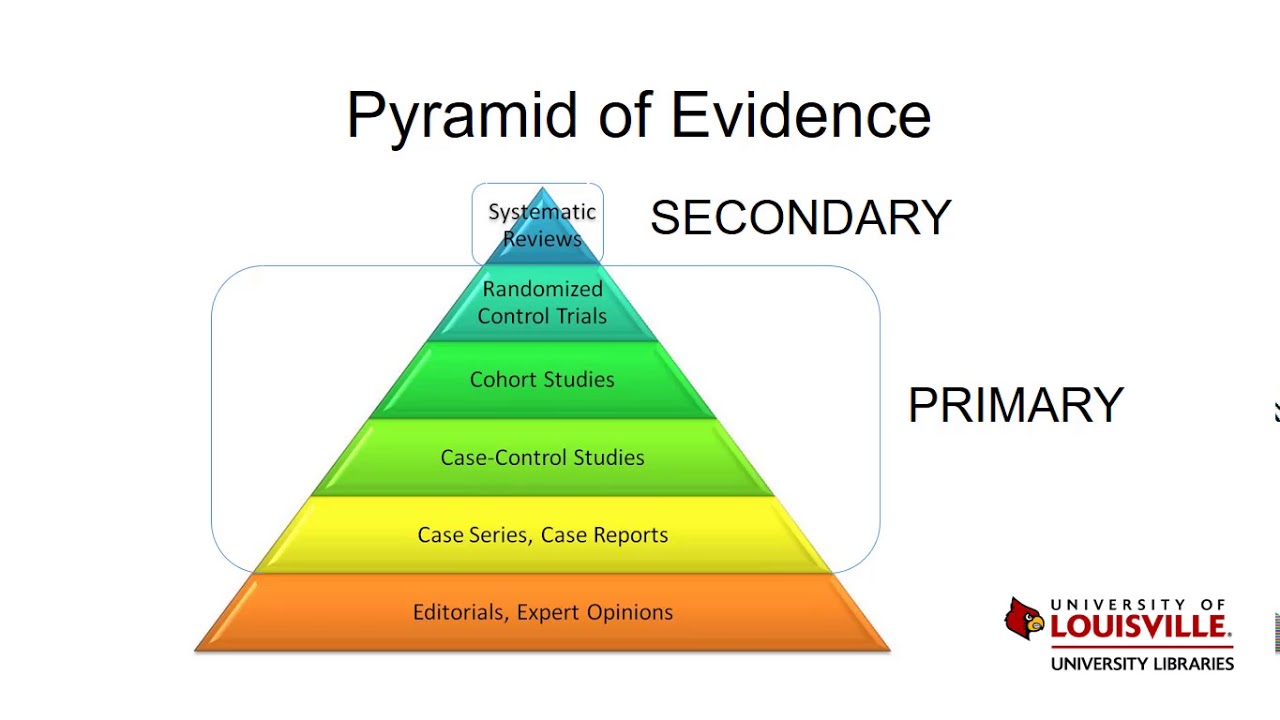
- 📊 The third module provides an overview of statistical concepts used in systematic reviews.
- ⚖️ Importance of randomized control trials (RCTs) in determining treatment effects highlighted.
- 🔗 Distinction made between controlled studies and observational studies in research methodology.
- ✍️ Key outcomes measured in RCTs include dichotomous and continuous outcomes.
- 📈 Explanation of meta-analysis and how it aggregates results from multiple studies.
- ⚠️ Importance of clinical significance over just statistical significance in interpreting results.
- 🔄 Discussion of relative risks, odds ratios, and mean differences in systematic reviews.
Q & A
What is the main focus of the project discussed in the modules?
-The project focuses on peer-reviewing Cochran systematic reviews and aims to train dietitians to participate in the production of these reviews.
What are the three modules provided in the training?
-The three modules cover an introduction to Cochran systematic reviews, how to provide peer-review feedback, and information about the statistics used in systematic reviews.
Why are randomized control trials emphasized in Cochran reviews?
-Randomized control trials are emphasized because they provide controlled conditions to determine the effects of treatments, minimizing biases that can occur in observational studies.
What are the two types of outcomes measured in systematic reviews?
-The two types of outcomes are dichotomous outcomes (e.g., yes/no events like fractures) and continuous outcomes (e.g., scores or measurements like pain levels).
What does a meta-analysis do?
-A meta-analysis combines results from multiple studies to provide a statistical overview of the effect of an intervention across those studies.
What is the significance of confidence intervals in research findings?
-Confidence intervals indicate the range in which the true effect is likely to fall, providing insight into the precision of the estimate and the possibility of variability in the results.
How are mean differences reported in systematic reviews?
-Mean differences are reported to show the average difference in outcomes between treatment and control groups, helping to interpret the clinical significance of results.
What does a relative risk of 1 indicate?
-A relative risk of 1 indicates no difference in risk between the treatment and control groups.
What is the role of clinical experience for dietitians in reviewing systematic reviews?
-Dietitians' clinical experiences are valuable for interpreting results and understanding how those results may affect healthcare decisions in practice.
Where can additional resources about statistics be found?
-Additional resources are available through the Bandelier series and Cochran modules, which cover topics such as meta-analysis and statistical measures like odds ratios and relative risks.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade Now5.0 / 5 (0 votes)