Thermal expansion for IGCSE, GCSE, GCE O level Physics
Summary
TLDRThis video explains thermal expansion, detailing how heating causes solids, liquids, and gases to expand due to increased kinetic energy of their particles. It contrasts the strong intermolecular forces in solids, which restrict expansion, with the weaker forces in liquids and gases, which allow for greater expansion and compression. The video highlights practical applications of thermal expansion, such as bimetallic strips in temperature switches, expansion gaps in railway tracks, and the behavior of liquids in thermometers. Everyday examples, like the inflation of car tires and the use of hot water to unscrew jar lids, illustrate the concept's relevance.
Takeaways
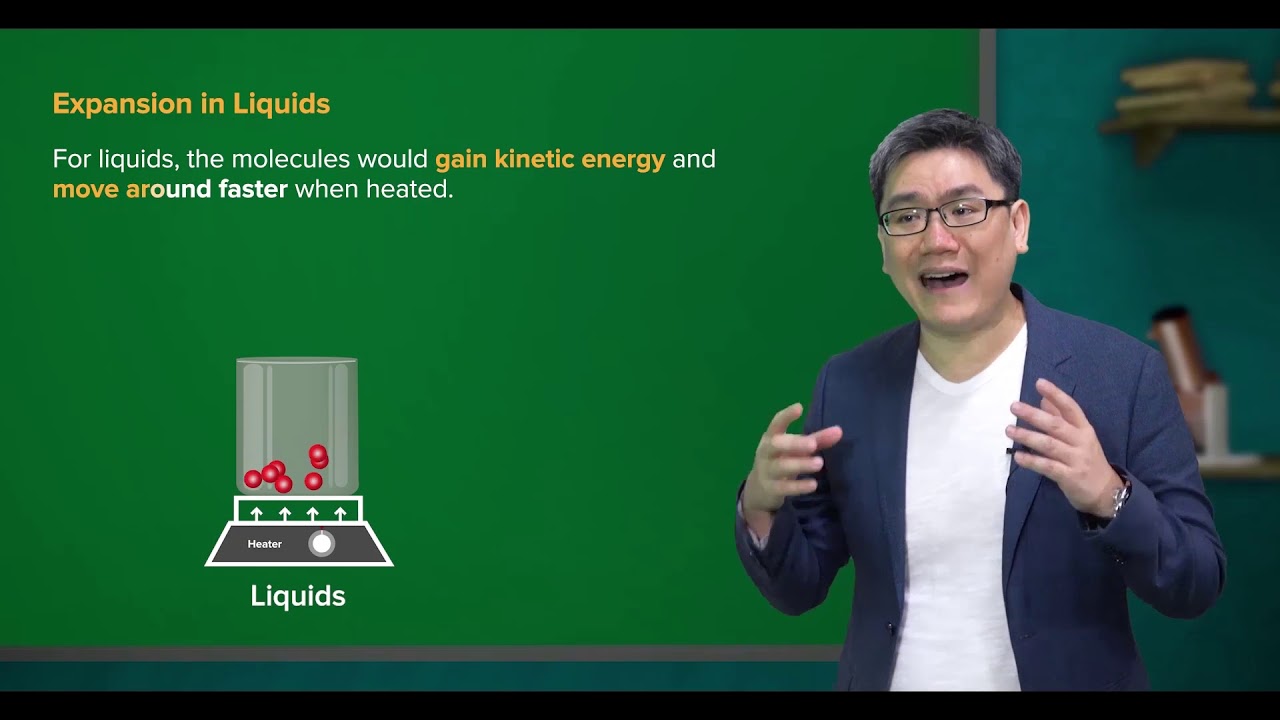
- 😀 Thermal expansion occurs when solids, liquids, or gases are heated, causing their particles to gain kinetic energy and move further apart.
- 😀 When cooled, substances experience contraction as particles lose kinetic energy and move closer together.
- 😀 Solids have strong intermolecular forces, making them the least prone to expansion and compression compared to liquids and gases.
- 😀 Liquids can expand and compress more easily than solids due to their relatively weaker intermolecular forces.
- 😀 Gases have very weak intermolecular forces, allowing them to expand and compress the most among the three states of matter.
- 😀 A bimetallic strip is used in temperature-activated switches; it bends when heated due to the different expansion rates of the two metals.
- 😀 Railway lines incorporate expansion gaps to prevent buckling during hot weather caused by thermal expansion.
- 😀 Thermometers utilize the thermal expansion of liquids, like mercury or alcohol, to measure temperature changes.
- 😀 Electrical cables are designed with slack to accommodate thermal expansion and prevent damage when temperatures change.
- 😀 Car tires require higher inflation pressure in the summer due to the expansion of air inside them when heated.
Q & A
What is thermal expansion?
-Thermal expansion is the increase in the volume of a substance when its temperature rises, caused by the gain in kinetic energy of its particles.
How do particles behave when a solid is heated?
-When a solid is heated, its particles gain kinetic energy, causing them to vibrate faster.
What happens to the particles of a gas when it is heated?
-The particles of a gas move faster when heated, leading to an increase in the distance between them.
Explain the difference between expansion and contraction.
-Expansion occurs when the particles gain kinetic energy and move apart, increasing volume, while contraction occurs when particles lose kinetic energy and come closer together, decreasing volume.
Which state of matter expands the most when heated?
-Gases expand the most when heated due to their weak intermolecular forces, allowing particles to move far apart.
What are bimetallic strips, and how are they used?
-Bimetallic strips are made of two different metals that expand at different rates. They are used in temperature-activated switches, like those that turn on a fan when the room gets too hot.
Why are expansion gaps important in railway lines?
-Expansion gaps in railway lines are important to prevent the tracks from buckling when temperatures rise and the metal expands.
How does a thermometer measure temperature?
-A thermometer measures temperature by using a liquid (such as mercury or alcohol) that expands and rises in a tube when heated, indicating the temperature.
Why must electrical cables have slack?
-Electrical cables must have slack to allow for thermal expansion and contraction, preventing breakage when they are cooled and contract.
What is a practical example of thermal expansion in everyday life?
-A practical example of thermal expansion is when hot water is run over a stuck jar lid, causing it to expand slightly, making it easier to unscrew.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
Cambridge IGCSE Physics | 2.13 Expansion and Contraction | GCSE O Level | My Second Teacher

Pemuaian Zat Padat, Zat Cair, dan Zat Gas (Pemuaian Panjang, Luas, dan Volume)

Particulate Nature of Matter and Changes of State

IGCSE Physics [Syllabus 2.1] Kinetic molecular model of matter

Fisika Kelas 7 - Ekspansi Thermal

FISIKA Kelas 11 - Suhu & Kalor (PART 1) | GIA Academy
5.0 / 5 (0 votes)