How Ionic Compounds Conduct Electricity - GCSE Chemistry | kayscience.com
Summary
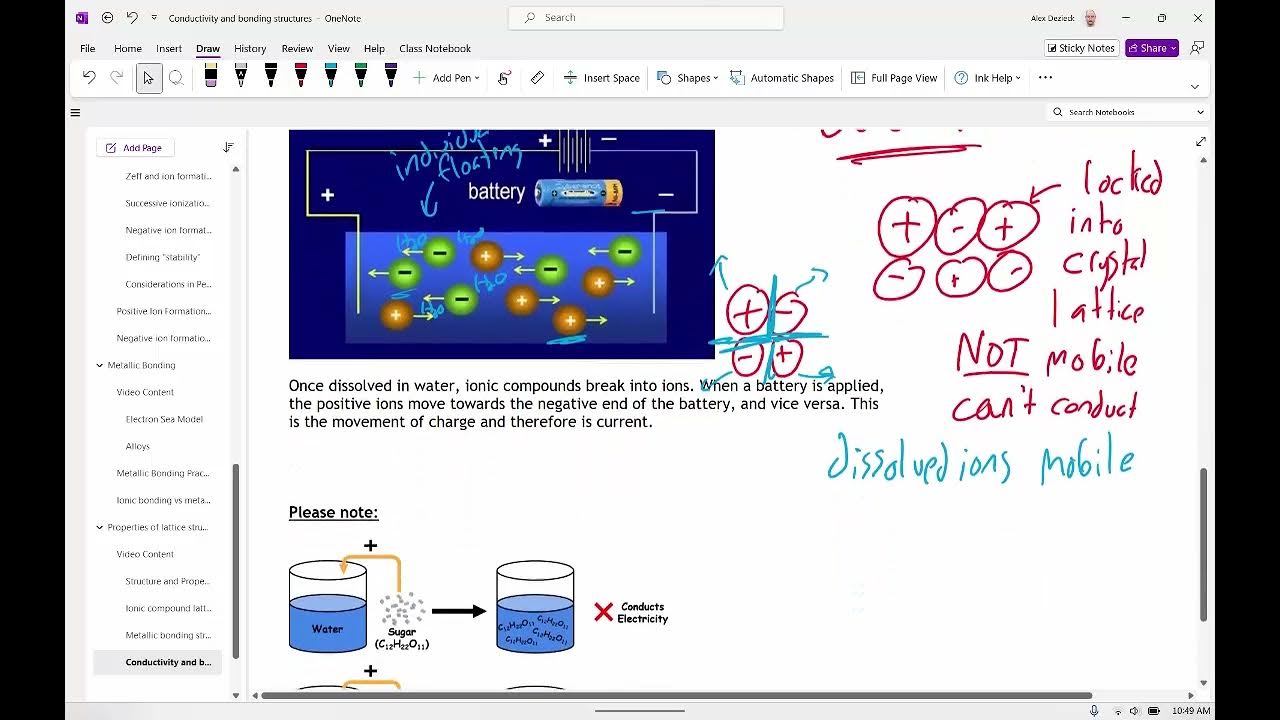
TLDRThis educational video demonstrates the conductivity of ionic compounds, highlighting the difference between solid and molten/aqueous states. It explains that solid ionic compounds cannot conduct electricity due to fixed ion positions, while molten or aqueous ionic compounds allow ions to move freely, enabling electrical current flow. The video details an experimental setup using a cell and electrodes to visually demonstrate these principles, reinforcing the concept that opposite charges attract. Viewers are encouraged to engage with the content and test their understanding through interactive questions.
Takeaways
- 😀 Solid ionic compounds cannot conduct electricity due to fixed ions held by strong ionic bonds.
- 🔄 Molten or aqueous ionic compounds can conduct electricity because their ions are free to move.
- ⚡ The flow of electricity in ionic compounds is due to the movement of ions, not electrons.
- 🔌 A power source, like a cell or power pack, is required to create an electric circuit for the experiment.
- ⚙️ Two electrodes are used: a positive electrode (inert) and a negative electrode (cathode).
- 🔴 Cations, represented by red circles, move toward the negative electrode during conduction.
- 🔵 Anions, represented by blue circles, move toward the positive electrode during conduction.
- 💡 To test for electric current, a bulb can be used to indicate if the current is flowing.
- 📊 An ammeter can provide readings to show the amount of current flowing in the circuit.
- 🧪 The shape of the molten or aqueous compound changes to fit its container, unlike solid ionic compounds.
Q & A
What is the primary purpose of the experiment described in the video?
-The experiment demonstrates that solid ionic compounds cannot conduct electricity, while molten or aqueous ionic compounds can.
What equipment is needed to conduct the experiment?
-You need a cell or power pack, wires, two electrodes, and a container for the molten or aqueous ionic compound.
How do the electrodes interact with the ions in the molten or aqueous ionic compound?
-Cations are attracted to the negative electrode (cathode), and anions are attracted to the positive electrode (anode), allowing an electric current to flow.
What happens to the ionic compound when it is in a solid state?
-In solid form, the ions are fixed in place by strong ionic bonds and cannot move, preventing the flow of electricity.
What is the mnemonic provided in the video to remember the relationship between cations and cathodes?
-The mnemonic is 'cation cathode,' which helps to associate cations with the negative electrode.
What does the video suggest using to prove that an electric current is flowing in the circuit?
-You can use a bulb to indicate that electricity is flowing or an ammeter to show readings of the current.
Why is it important to understand the movement of ions in this experiment?
-Understanding ion movement is crucial because it directly relates to how electricity is conducted in different states of ionic compounds.
What should you do if you struggle to answer the practice questions provided in the video?
-If you have difficulty, you can rewatch the video to better understand the concepts before attempting the questions again.
How does the structure of solid ionic compounds affect their electrical conductivity?
-The rigid structure of solid ionic compounds restricts ion movement, which is necessary for electrical conductivity, leading to no current flow.
Where can viewers find more resources related to the topic discussed in the video?
-Viewers can visit K Science's website for more videos, worksheets, and quizzes related to the subject.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
5.0 / 5 (0 votes)