Mecanismo de hidrólisis del ATP | Energía y enzimas | Biología | Khan Academy en Español
Summary
TLDRThis video explains the vital role of ATP (adenosine triphosphate) as the energy currency of cells, focusing on the hydrolysis process where ATP converts to ADP and a phosphate group. It details how this spontaneous reaction releases about 30.5 kJ/mol of energy but requires the assistance of enzymes called ATPases to overcome the activation energy barrier. The ATPases facilitate the reaction by surrounding ATP with positive ions, enabling the hydrolysis to occur efficiently. The released energy is typically harnessed for cellular functions, including phosphorylation of molecules and other essential processes.
Takeaways
- ⚡ ATP (adenosine triphosphate) is the energy currency of cells, crucial for energy transfer.
- 🔋 ATP hydrolysis releases approximately 30.5 kJ/mol of free energy, making it a spontaneous reaction.
- ⛰️ The reaction requires overcoming an activation energy barrier, which is illustrated graphically.
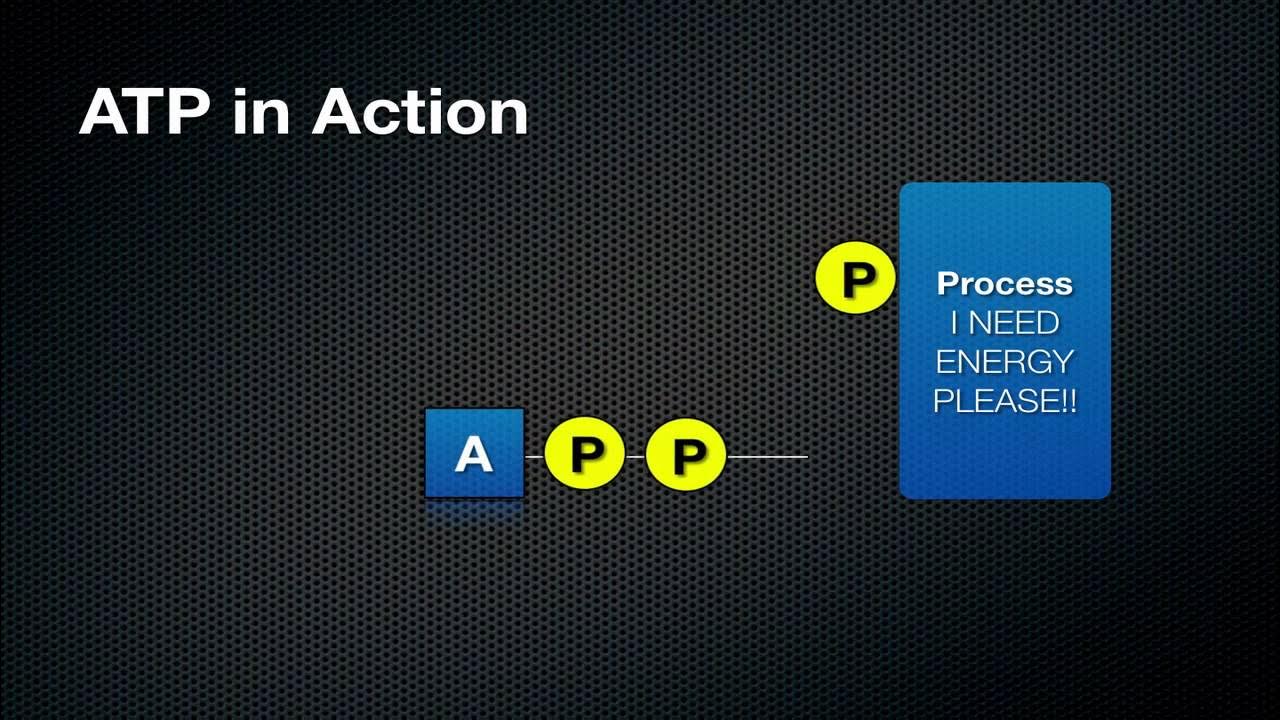
- 💧 Hydrolysis involves the reaction of ATP with water, facilitating the breakdown into ADP and a phosphate group.
- 🔄 ATPases are specialized enzymes that lower the activation energy required for ATP hydrolysis.
- ⚖️ ATPases stabilize negative charges in ATP by surrounding it with positive ions, enabling efficient reactions.
- 🧪 The nucleophilic attack by water on the phosphorus atom is key to ATP hydrolysis.
- 💥 The energy released from ATP hydrolysis is used for various cellular processes, such as phosphorylation and transport.
- 🌡️ Some energy from the reaction may dissipate as heat, but most is utilized productively in the cell.
- 🔍 The term 'hydrolysis' specifically refers to the use of water to remove a phosphate group from ATP.
Q & A
What is ATP and why is it important for cells?
-ATP, or adenosine triphosphate, is known as the energy currency of the cell. It provides the necessary energy for various cellular processes.
What happens during the hydrolysis of ATP?
-During the hydrolysis of ATP, it breaks down into ADP (adenosine diphosphate) and an inorganic phosphate group, releasing energy in the process.
Why is the hydrolysis of ATP considered a spontaneous reaction?
-The hydrolysis of ATP is spontaneous because it results in a decrease in free energy, typically around -30.5 kJ/mol, indicating that the reaction can occur without the input of additional energy.
What role does the activation energy play in ATP hydrolysis?
-Activation energy is the energy barrier that must be overcome for the reaction to proceed. ATP hydrolysis requires this energy unless it is catalyzed by enzymes.
How do enzymes facilitate the hydrolysis of ATP?
-Enzymes, specifically ATPases, lower the activation energy required for the hydrolysis of ATP by stabilizing the transition state and allowing the reaction to occur more readily.
What is the function of water in the hydrolysis of ATP?
-Water participates in the hydrolysis reaction by attacking the phosphorus atom in ATP, resulting in the formation of ADP and an inorganic phosphate.
What are the consequences of ATP hydrolysis for cellular functions?
-The energy released from ATP hydrolysis is often used to phosphorylate other molecules, enabling various cellular processes such as active transport and metabolic reactions.
What is a nucleophilic attack, and how does it relate to ATP hydrolysis?
-A nucleophilic attack occurs when a nucleophile, such as water in this case, donates a pair of electrons to an electrophile, which is the phosphorus atom in ATP, facilitating the breakdown of ATP.
What happens to the released energy during ATP hydrolysis?
-The energy released during ATP hydrolysis is typically harnessed by the cell for work, such as transporting molecules against their concentration gradient or changing the conformation of proteins.
What is meant by the term 'hydrolysis,' and why is it used in this context?
-Hydrolysis refers to the chemical breakdown of a compound due to reaction with water. In the context of ATP, it describes the process of breaking ATP down into ADP and inorganic phosphate using water.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade Now5.0 / 5 (0 votes)