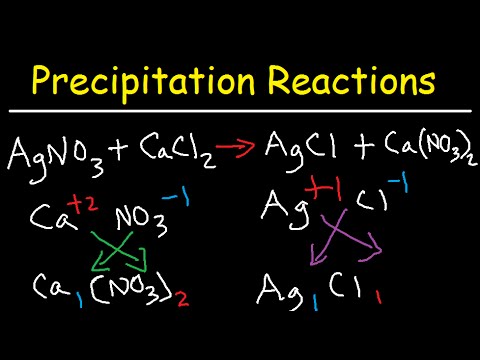
CHEM 1B Intro Qualitative Analysis 2 TAA Precipitation
Summary
TLDRThe video demonstrates a step-by-step process for separating and identifying different metal ions in a solution through sequential precipitation. Starting with the addition of HCl to isolate silver chloride, the procedure moves to precipitating copper(II) sulfide and iron(III) sulfide using thioacetamide. The importance of maintaining an acidic environment for selective precipitation is highlighted, with checks using pH paper and ammonia for adjustments. The procedure concludes with centrifugation and proper waste disposal. The focus is on teaching chemistry students the strategy behind sequential cation separation, providing both hands-on and theoretical insights.
Takeaways
- 🔬 The first step involves adding HCl to precipitate silver chloride, which is then collected as a pellet.
- 🧪 The supernatant contains copper (II) and iron (III) ions, which will be precipitated as sulfides.
- 🔍 Sulfide wasn't used initially to avoid precipitating silver ions as silver sulfide, which would complicate separation.
- 🧴 Thioacetamide is used to selectively precipitate copper (II) sulfide, leaving iron (III) ions in the solution for further analysis.
- ⚗️ Maintaining the solution at a pH of 0.5 is critical to selectively precipitate copper (II) sulfide over iron (III) sulfide.
- 🧪 pH adjustments are made using ammonia, typically 1M or 6M solutions, to ensure the correct acidity or basicity for selective precipitations.
- 👨🔬 After adding thioacetamide and boiling the solution, a dark precipitate forms, which is confirmed to be copper (II) sulfide.
- 🧫 The precipitate is centrifuged to separate copper sulfide, and the supernatant, which contains iron (III) ions, is decanted for further steps.
- 📊 After copper is separated, ammonia is added to make the solution basic, precipitating iron (III) sulfide.
- 🧪 A litmus test confirms the basicity of the solution, ensuring the iron (III) ions are precipitated as iron sulfide, which is then centrifuged and separated for further analysis.
Q & A
What is the purpose of adding HCl in the first step of the procedure?
-The purpose of adding HCl is to precipitate silver ions as silver chloride, which can then be separated from the solution.
Why wasn't sulfide added first to precipitate the silver ions?
-Sulfide wasn't added first because it would have also precipitated silver ions as silver sulfide, which would have interfered with the selective precipitation process.
What is the function of thioacetamide in this procedure?
-Thioacetamide is used to generate sulfide ions in the solution, which helps selectively precipitate cations such as copper (II) as copper (II) sulfide.
Why must the solution be kept at a pH of 0.5 when precipitating copper (II) sulfide?
-Maintaining a pH of 0.5 ensures that copper (II) ions selectively precipitate as copper (II) sulfide, while keeping the iron (III) ions in solution for further separation.
What role does ammonia play in adjusting the pH of the solution?
-Ammonia is added to neutralize excess acid and raise the pH of the solution, making sure the conditions are right for selective precipitation.
How is the pH of the solution checked during the procedure?
-The pH is checked using a specially coated pH paper. A stir rod is dipped into the solution, and then the solution is dabbed onto the pH paper for measurement.
Why is it necessary to work in a fume hood during the precipitation process?
-It is necessary to work in a fume hood because the chemicals, especially sulfides, produce strong and unpleasant odors that must be contained for safety.
What indicates the successful precipitation of copper (II) sulfide in the boiling water bath?
-The solution turns dark as copper (II) sulfide precipitates, signaling that the copper ions have successfully been precipitated.
How is the precipitate of copper (II) sulfide separated from the supernatant?
-The precipitate is separated by centrifuging the solution, which compacts the precipitate into a pellet, allowing the supernatant to be decanted off.
What is done to ensure all copper ions have been precipitated from the solution?
-After decanting, a few more drops of thioacetamide are added to the supernatant to confirm that no additional copper ions remain in the solution.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
5.0 / 5 (0 votes)