Why are ionic compounds soluble in water?
Summary
TLDRThe video explains why many ionic compounds or salts are soluble in water, highlighting the interaction between ions in the ionic lattice and water molecules. Water's uneven charge distribution, with oxygen being more electronegative than hydrogen, creates a permanent dipole. When salts like sodium chloride dissolve, water molecules surround the ions, forming ion-dipole attractions. This dissolution process is driven by both energy released from forming new attractions and increased entropy, as the structured lattice breaks into a more disordered solution. The video also emphasizes the neutrality of ions once hydrated.
Takeaways
- 💧 Many ionic compounds or salts are soluble in water due to the attraction between the ions and water molecules.
- ⚛️ Water molecules have an uneven distribution of electric charge, with oxygen being more electronegative than hydrogen.
- 🧲 The oxygen in a water molecule pulls electrons more strongly than the hydrogen, creating a permanent dipole.
- 🧑🔬 The oxygen end of a water molecule has a negative charge (delta negative), while the hydrogen end has a positive charge (delta positive).
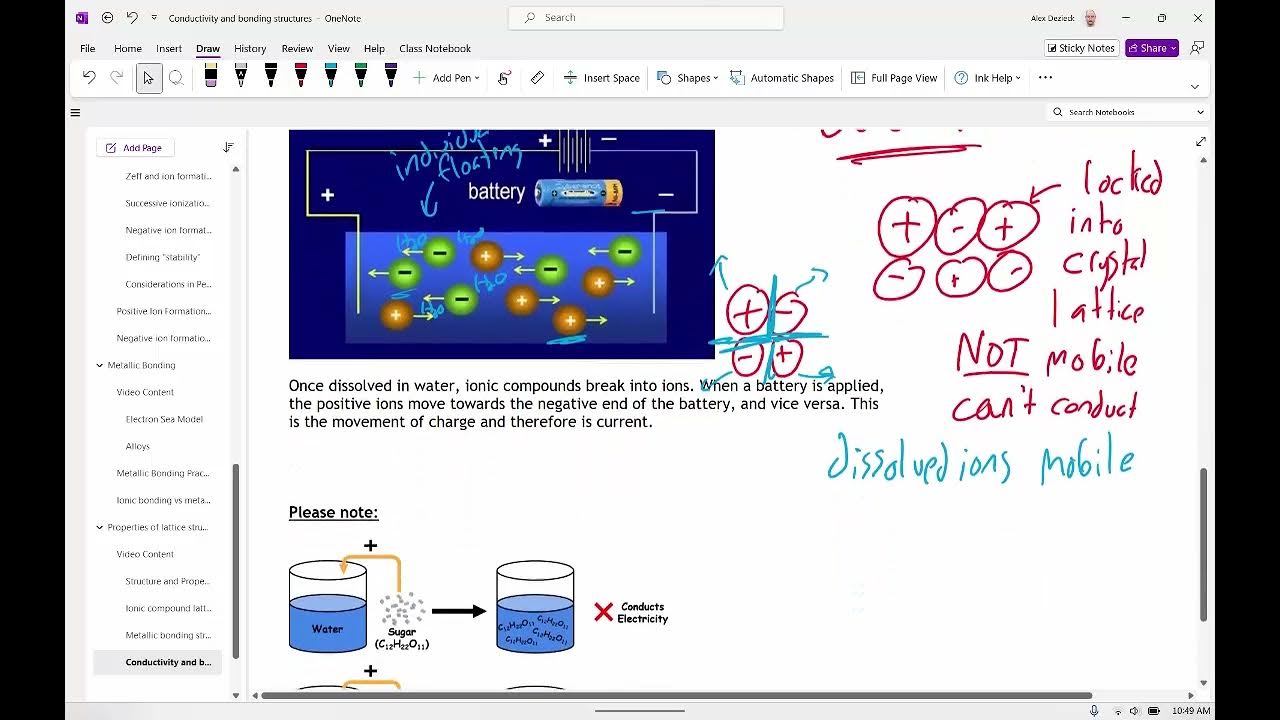
- ⚡ When ionic salts, like sodium chloride, are added to water, water molecules are attracted to the ions, breaking the ionic lattice.
- 🔄 The attraction between water molecules and ions is called an ion-dipole interaction, which helps dissolve the salt.
- 🌊 Water molecules form layers around ions, releasing energy that partially balances the energy needed to break the ionic lattice.
- 🔋 The dissolving process is also driven by an increase in entropy, as the system becomes more disordered when the ions move freely in solution.
- 🌍 Once hydrated, ions are assumed to be electrically neutral, no longer feeling the pull of each other's charge.
- 📚 This explanation helps understand why many ionic compounds dissolve in water due to both ion-dipole interactions and entropy.
Q & A
Why are many ionic compounds soluble in water?
-Many ionic compounds are soluble in water due to the attraction between the ions and the polar water molecules. The polar nature of water allows it to interact with the ions, leading to the dissolution of the ionic compound.
What is the distribution of electric charge within a water molecule?
-The electric charge within a water molecule is not evenly distributed. The oxygen atom, having more protons, exerts a stronger pull on the shared electrons, resulting in a partial negative charge on the oxygen and a partial positive charge on the hydrogen atoms.
Why is oxygen considered electronegative in a water molecule?
-Oxygen is considered electronegative because it has a greater ability to attract the shared pairs of electrons towards itself due to having more protons in its nucleus compared to hydrogen.
What is the significance of the term 'permanent dipole' in the context of water molecules?
-A 'permanent dipole' refers to the unequal distribution of charge in a water molecule, with a negative charge centered around the oxygen and a positive charge around the hydrogens, creating a polar molecule.
How do water molecules interact with ionic compounds like sodium chloride?
-Water molecules interact with ionic compounds through ion-dipole attractions. The oxygen end of water is attracted to the positively charged sodium ions, while the hydrogen end is attracted to the negatively charged chloride ions.
What is an ion-dipole attraction?
-An ion-dipole attraction is the force of attraction between an ion (charged particle) and the oppositely charged end of a polar molecule, such as the interaction between water molecules and ions in a salt like sodium chloride.
How does the process of dissolving ionic compounds in water relate to energy changes?
-The energy released when new attractions are formed between water molecules and ions can balance the energy required to break the ionic lattice, making the dissolution process energetically favorable.
What role does entropy play in the dissolution of ionic compounds in water?
-Entropy, a measure of disorder in a system, increases during the dissolution of ionic compounds in water. The process is favored as it leads to a more chaotic and disordered state, with ions surrounded by hydration shells.
Why is the increase in entropy favorable for the dissolution of ionic compounds?
-The increase in entropy is favorable because it corresponds to a more disordered state, which is typically more stable and energetically favorable according to the second law of thermodynamics.
What happens to the ions once they are hydrated in water?
-Once ions are hydrated, they are surrounded by water molecules, which effectively neutralize their charge, preventing them from re-forming the ionic lattice and thus remaining dissolved.
How can the concepts discussed in the script be applied to study aids or educational materials?
-The concepts can be applied to educational materials by creating visual aids, such as diagrams of water molecule polarity and ion-dipole interactions, as well as examples and exercises that demonstrate the principles of solubility and entropy.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Dissolution Of NaCl In Water

Properties of Ionic Substances | Properties of Matter | Chemistry | FuseSchool
Conductivity and Reflectivity

Processes Involved in Dissolution of Ionic Compounds // HSC Chemistry

Properties of Ionic Compounds

Giant Ionic Structures or Lattices | Properties of Matter | Chemistry | FuseSchool
5.0 / 5 (0 votes)