2.5 The Periodic Table + the Atom
Summary
TLDRThis video segment links the Bohr model of the atom with the periodic table. The instructors explain how electrons fill different energy levels or shells, and focus on the importance of the outermost shell, known as the valence shell, in determining an atom’s interactions. They highlight that atoms strive for stability by gaining or losing electrons to achieve a full outer shell of eight electrons, known as the octet rule. The periodic table provides clues about how many electrons atoms in various groups will lose or gain to become stable, which plays a crucial role in bonding.
Takeaways
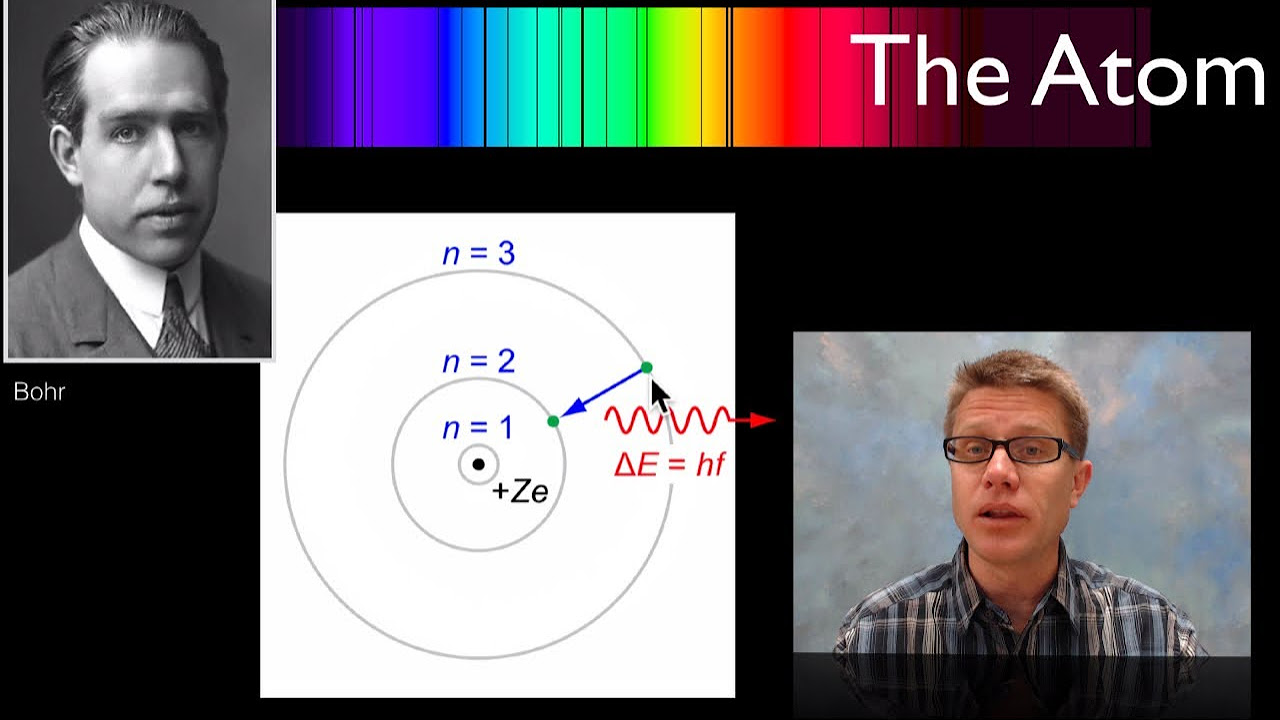
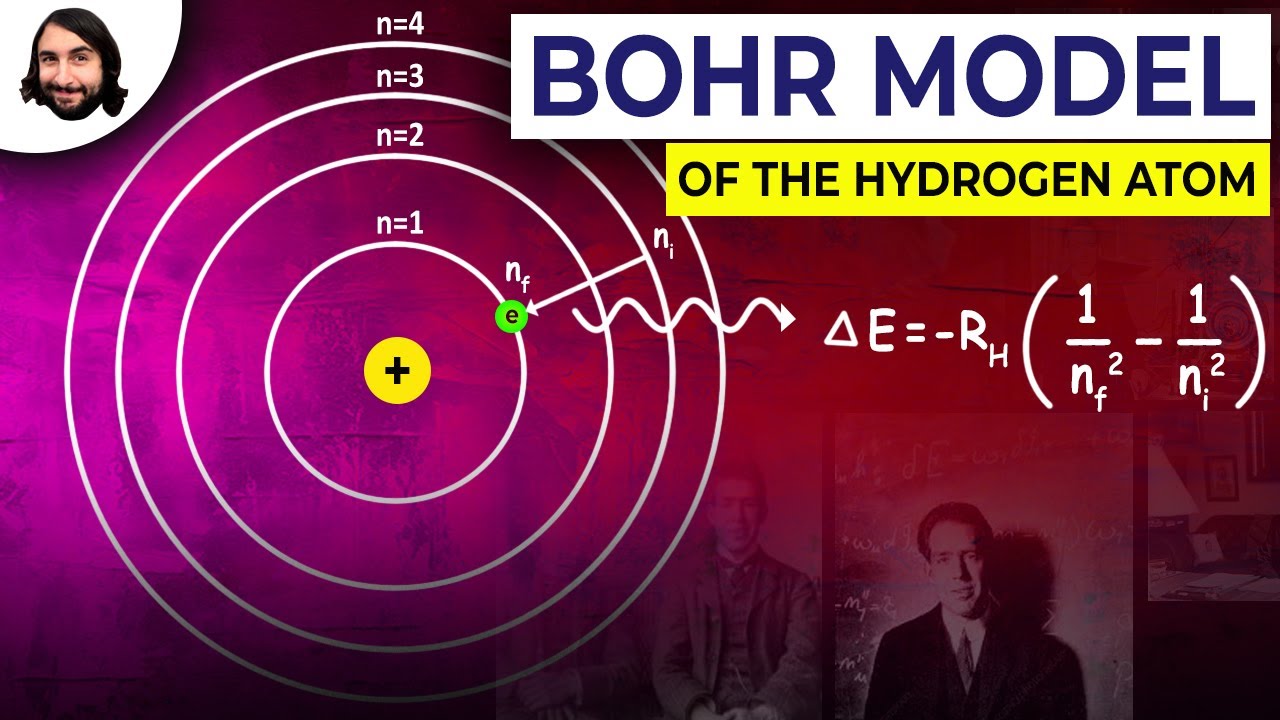
- 🔬 The Bohr model explains that atoms have shells of electrons, with the first shell holding 2 electrons and subsequent shells holding 8.
- ⚛️ Atoms interact through their outermost energy level, also known as the valence shell, which plays a key role in bonding.
- 📊 The periodic table is structured to reflect the behavior of electrons, with elements grouped by their number of valence electrons.
- 🔋 Elements in column 1 of the periodic table have a +1 charge because they lose 1 electron, while those in column 2 have a +2 charge due to losing 2 electrons.
- ⚖️ Atoms want to achieve a stable configuration with 8 valence electrons, referred to as an 'octet,' and they can gain or lose electrons to reach this state.
- 😃 The elements in group 18, the noble gases, are stable and do not readily form bonds because they already have 8 valence electrons.
- 🔗 Atoms with fewer than 8 electrons in their valence shell will either gain or lose electrons to achieve stability. Atoms with 1-3 valence electrons tend to lose them, while those with 5-7 tend to gain them.
- ➕ Atoms that lose electrons become positively charged, while those that gain electrons become negatively charged.
- 📥 Group 15 elements tend to gain 3 electrons and acquire a -3 charge, while group 16 gains 2 electrons with a -2 charge, and group 17 gains 1 electron with a -1 charge.
- 🧲 The periodic table not only provides information on atomic structure but also helps in understanding how atoms bond by indicating their charge when they gain or lose electrons.
Q & A
What is the Bohr model of the atom?
-The Bohr model describes atoms as having electrons in specific shells or energy levels around the nucleus. The first shell holds 2 electrons, and subsequent shells hold 8 or more electrons.
How many electrons can the first and second shells hold in the Bohr model?
-The first shell can hold up to 2 electrons, while the second shell can hold up to 8 electrons.
What is meant by the term 'valence electrons'?
-Valence electrons are the electrons in the outermost shell of an atom. These electrons are responsible for chemical bonding and interactions with other atoms.
Why is the valence shell important?
-The valence shell is important because it is the outermost shell and is the part of the atom that interacts with other atoms during chemical reactions.
What is the 'octet rule' mentioned in the script?
-The octet rule refers to the tendency of atoms to seek a stable configuration with 8 electrons in their valence shell. Atoms gain, lose, or share electrons to achieve this stable configuration.
Why are noble gases considered 'happy' or stable?
-Noble gases are stable because they naturally have a full outer shell with 8 valence electrons, making them unreactive or 'happy' as they do not need to gain or lose electrons.
What happens when an atom has only 1 or a few valence electrons?
-Atoms with 1 or a few valence electrons, like those in group 1 of the periodic table, tend to lose electrons easily to achieve a full outer shell, often becoming positively charged ions.
How do atoms in groups 1, 2, and 3 of the periodic table become stable?
-Atoms in groups 1, 2, and 3 become stable by losing 1, 2, or 3 electrons, respectively, to achieve a full inner shell of 8 electrons. This loss of electrons results in a positive charge.
How do atoms in groups 5, 6, and 7 of the periodic table become stable?
-Atoms in groups 5, 6, and 7 become stable by gaining 3, 2, or 1 electrons, respectively, to achieve 8 valence electrons, leading to a negative charge.
Why are the charges of ions important in chemical bonding?
-The charges of ions are important because they determine how atoms bond. Positively charged ions (cations) attract negatively charged ions (anions), forming ionic bonds.
Why are elements in the middle of the periodic table (transition metals) skipped in this discussion?
-The transition metals are skipped in this discussion because their electron configurations are more complex, and they do not follow the simple rules for gaining or losing electrons like the main group elements.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade Now5.0 / 5 (0 votes)