Spontaneity and redox reactions | Redox reactions and electrochemistry | Chemistry | Khan Academy
Summary
TLDRThe video script discusses predicting whether lead(II) can oxidize solid aluminum or copper under standard conditions and calculating the standard cell potentials for these reactions at 25°C. It uses the diagonal rule from the standard reduction potential table to predict that lead(II) can oxidize aluminum but not copper. The script confirms these predictions by calculating the standard cell potentials, finding a positive potential for aluminum oxidation and a negative potential for copper, indicating that the former is a spontaneous reaction while the latter is not.
Takeaways
- 🔬 The goal is to predict if Pb2+ can oxidize solid Al or Cu and calculate the standard cell potentials at 25°C.
- 📊 A standard reduction potential table is used to determine if Pb2+ can oxidize other metals.
- 📉 An oxidizing agent like Pb2+ can oxidize reducing agents listed below it in the table.
- ➡️ Lead two plus (Pb2+) can oxidize aluminum (Al) as Al is below Pb2+ in the table, confirmed by the diagonal rule.
- ❌ Lead two plus (Pb2+) cannot oxidize copper (Cu) as Cu is above Pb2+ in the table.
- 🔋 The standard reduction potential for Pb2+ to Pb is -0.13 volts.
- 🔌 The standard oxidation potential for Al to Al3+ is +1.66 volts when reversed from reduction.
- 🔄 To balance the overall reaction, the half-reactions are multiplied to equalize the electrons transferred.
- 🔄 The standard cell potential is calculated by adding the reduction and oxidation potentials.
- 💡 A positive standard cell potential indicates a spontaneous reaction, confirming Pb2+ can oxidize Al.
- 📉 A negative standard cell potential for the reaction with Cu indicates a non-spontaneous reaction, confirming Pb2+ cannot oxidize Cu.
Q & A
What is the goal of the experiment described in the transcript?
-The goal is to predict whether lead (II) can oxidize solid aluminum or solid copper under standard state conditions and to calculate the standard cell potentials for each reaction at 25 degrees Celsius.
What is the significance of the standard reduction potential table in this context?
-The standard reduction potential table is used to determine if lead (II) can act as an oxidizing agent for aluminum and copper by comparing their positions relative to lead (II) on the table.
According to the transcript, can lead (II) oxidize aluminum?
-Yes, lead (II) can oxidize aluminum because aluminum is listed below lead (II) on the standard reduction potential table, indicating it is a stronger reducing agent.
What is the diagonal rule mentioned in the transcript?
-The diagonal rule is a method used to predict whether a reaction will occur by drawing a diagonal line from the oxidizing agent to the reducing agent on the standard reduction potential table.
Why can't lead (II) oxidize copper according to the transcript?
-Lead (II) cannot oxidize copper because copper is listed above lead (II) on the standard reduction potential table, meaning it is a weaker reducing agent.
How is the standard cell potential calculated for the reaction between lead (II) and aluminum?
-The standard cell potential is calculated by adding the standard reduction potential of lead (II) (-0.13 V) to the standard oxidation potential of aluminum (+1.66 V), resulting in a positive value of +1.53 V, indicating a spontaneous reaction.
What does a positive standard cell potential signify?
-A positive standard cell potential signifies that the reaction is spontaneous, meaning it will occur without the need for external energy.
How is the standard oxidation potential for aluminum determined in the transcript?
-The standard oxidation potential for aluminum is determined by reversing the standard reduction potential for aluminum (-1.66 V) and changing its sign to positive (+1.66 V).
What is the standard cell potential for the reaction between lead (II) and copper?
-The standard cell potential for the reaction between lead (II) and copper is calculated to be -0.47 V, indicating a non-spontaneous reaction.
Why is the reaction between lead (II) and copper non-spontaneous?
-The reaction is non-spontaneous because the calculated standard cell potential is negative (-0.47 V), which means the reaction does not have a natural tendency to occur.
How does the activity series relate to the standard reduction potentials?
-The activity series can be explained by looking at standard reduction potentials; metals with more negative standard reduction potentials are more easily oxidized and are considered more active.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

QUIMICA REDOX T4.7 Potencial estándar de reducción de las semireacciones

ELEKTROKIMIA | SEL VOLTA | SEL GALVANI | POTENSIAL SEL | REDOKS

PERUBAHAN ENTALPI STANDAR ( BAB TERMOKIMA - MAPEL KIMIA KELAS 11 )
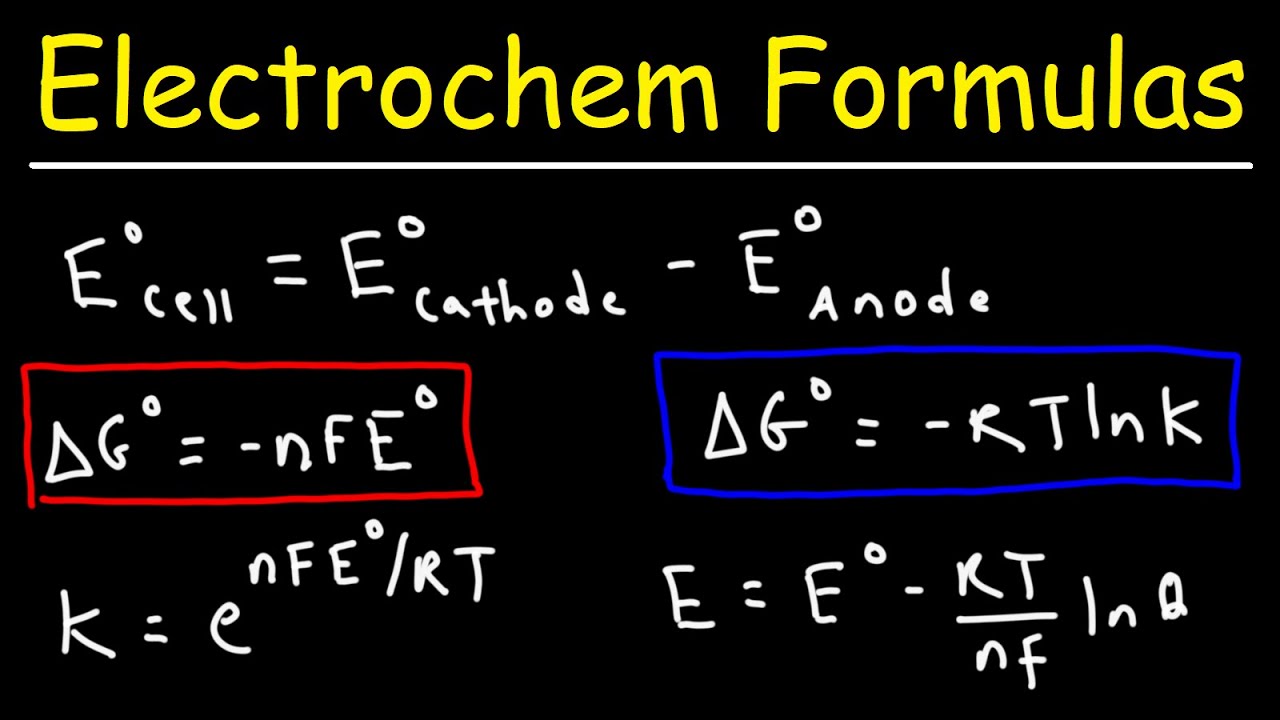
Electrochemistry Formulas - Gibbs Free Energy, Equilibrium K, Cell Potential, Nernst Equation

Pembahasan Soal Sel Volta Kimia Kelas 12 #Part 1

TERMOKIMIA part 2- jenis-jenis perubahan entalpi standar Kimia kelas 11 semester 1
5.0 / 5 (0 votes)