Nuclear Half Life: Calculations
Summary
TLDRThis educational script explains the concept of half-life in radioactive decay using examples of radium to radon, tritium to helium-3, and thallium to lead. It covers calculations for remaining mass, percentage, and fraction after specific half-lives, emphasizing a step-by-step approach. The script also introduces the process of determining half-life duration when given initial and final masses over a known time.
Takeaways
- 📚 The half-life of radium is 11 days, and after 44 days (4 half-lives), 7.5 grams of a 120-gram sample would remain.
- 🔢 After 44 days, 6.25% of the original radium sample would be left.
- 🔄 The fraction remaining after 44 days of radium's half-life is 1/16, which is equivalent to 6.25%.
- 🕒 Tritium has a half-life of 12.3 years, and it takes 61.5 years for an 80-gram sample to decay to 2.5 grams.
- 📉 If 3.125% of tritium remains, it also takes 61.5 years for the decay, indicating 5 half-lives have passed.
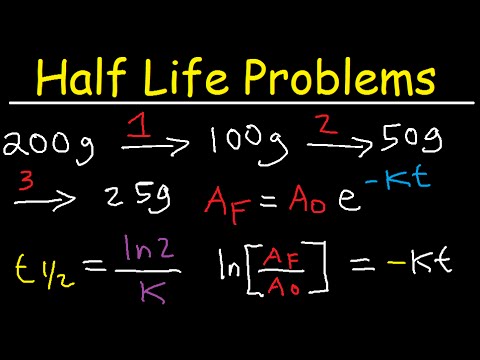
- 🔢 To find the half-life of thallium-207, which decays to lead, start with 200 grams and observe it takes 20 minutes to reduce to 12.5 grams, indicating a half-life of 5 minutes.
- 📊 The process of decay can be visualized through charts showing the amount of substance left after each half-life.
- 🧮 Half-life problems can be solved using simple division for time or multiplication for fractions, but more complex problems may require exponents and logarithms.
- ⏳ The concept of half-life is crucial for understanding radioactive decay and can be applied to various isotopes like radium, tritium, and thallium.
- 🔬 Radioactive decay problems can be approached by considering the physical amount, percentage, or fraction remaining after a given number of half-lives.
Q & A
What is the half-life of radium-222 decaying into radon?
-The half-life of radium-222 decaying into radon is 11 days.
If you start with 120 grams of radium, how much will be left after 44 days?
-After 44 days, which is equivalent to 4 half-lives, 7.5 grams of radium will be left.
How can you calculate the percentage of a substance left after a certain number of half-lives?
-You start at 100% and reduce it by half for each half-life that passes. After 4 half-lives of radium-222, 6.25% of the original amount will be left.
What fraction of the original amount of radium-222 will be left after 44 days?
-After 4 half-lives, 1/16 of the original amount of radium-222 will be left.
How long does it take for 80 grams of tritium to decay to 2.5 grams?
-It takes 61.5 years for 80 grams of tritium to decay to 2.5 grams, considering each half-life is 12.3 years.
What is the half-life of tritium decaying into helium-3?
-The half-life of tritium decaying into helium-3 is 12.3 years.
If you start with 200 grams of thallium-207, how long does it take for it to decay to 12.5 grams?
-It takes 20 minutes for 200 grams of thallium-207 to decay to 12.5 grams.
What is the half-life of thallium-207 decaying into lead?
-The half-life of thallium-207 decaying into lead is 5 minutes.
How can you determine the half-life of a substance if you know the initial and final amounts and the time taken for the decay?
-You can determine the half-life by dividing the total time taken by the number of half-lives that occurred, as shown with thallium-207 where 20 minutes divided by 4 half-lives equals a 5-minute half-life.
What is the relationship between the number of half-lives and the remaining percentage of a substance?
-The remaining percentage of a substance after a certain number of half-lives is the original percentage divided by 2 for each half-life that has passed.
How can you calculate the time it takes for a substance to decay to a certain fraction of its original amount?
-You multiply the half-life of the substance by the number of half-lives needed to reach the desired fraction, as shown with tritium where 12.3 years times 5 half-lives equals 61.5 years.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Half Life / Separuh Hayat

GCSE Physics Revision "Half Life"

Half life | Radioactivity | Physics | FuseSchool

What is radioactivity and half-life? | Nuclear Physics | Visual Explanation

Nuclear Fission and Radioactivity - Part 3 of 3
Half Life Chemistry Problems - Nuclear Radioactive Decay Calculations Practice Examples
5.0 / 5 (0 votes)