Rates of Reactions - Part 1 | Reactions | Chemistry | FuseSchool
Summary
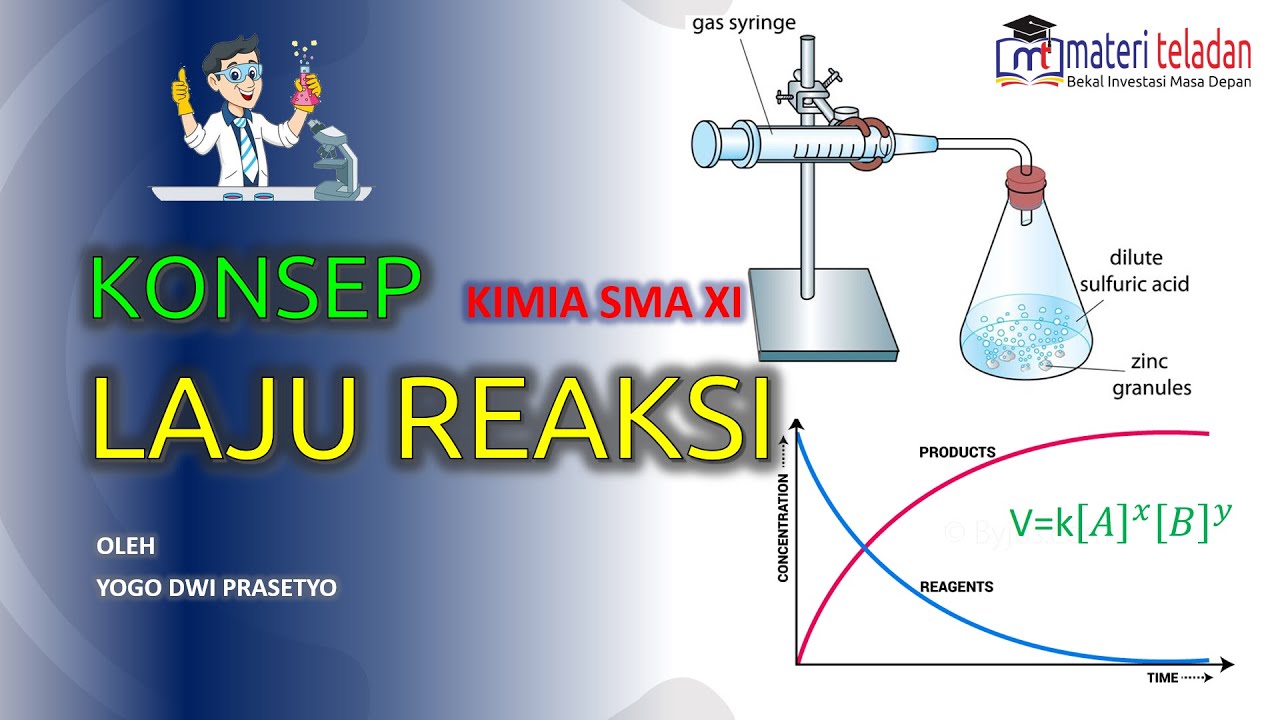
TLDRThis video explains the concept of reaction rate, which measures how quickly reactants change into products in a chemical reaction. It covers two methods for measuring reaction rates: the first method tracks how fast reactants decrease, using a reaction between marble chips (calcium carbonate) and hydrochloric acid as an example, where mass loss is recorded. The second method involves measuring the volume of a gas product (carbon dioxide). The video concludes by emphasizing that the rate of reaction typically decreases over time, whether measuring reactants or products.
Takeaways
- 😀 Reaction rate refers to how quickly reactants change into products in a chemical reaction.
- 😀 The rate of a chemical reaction can be measured by observing either the decrease of reactants or the increase of products.
- 😀 One way to measure reaction rate is by tracking how quickly the reactants decrease in mass during the reaction.
- 😀 The example of calcium carbonate reacting with hydrochloric acid demonstrates how the mass of reactants decreases as carbon dioxide is produced.
- 😀 A graph can be plotted showing the mass of reactants versus time to visually track the reaction rate.
- 😀 The rate of reaction is determined by the gradient (y/x) of the mass-time graph at different points in the reaction.
- 😀 Another way to measure reaction rate is by observing the increase in the volume of products, such as the carbon dioxide gas produced in the calcium carbonate reaction.
- 😀 A graph of volume of gas produced versus time also helps track the rate of reaction.
- 😀 As the reaction proceeds, both the decrease in reactants and the increase in products slow down over time.
- 😀 The rate of reaction typically decreases as the reaction continues, meaning it slows down as the reaction progresses.
Q & A
What is reaction rate?
-Reaction rate is the measure of how quickly the reactants in a reaction change into the products of the reaction.
How can reaction rate be measured?
-Reaction rate can be measured in two ways: by measuring how quickly the reactants decrease, or by measuring how quickly the products increase.
What does measuring the decrease in reactants tell us about the reaction rate?
-Measuring the decrease in reactants tells us how quickly the substances on the left side of the reaction equation (reactants) are consumed in the reaction.
In the marble chips and hydrochloric acid reaction, what are the reactants and products?
-The reactants are calcium carbonate (marble chips) and hydrochloric acid, and the products are calcium chloride, water, and carbon dioxide.
How can the decrease in mass of the reactants be measured?
-The decrease in mass of the reactants can be measured using an apparatus that tracks the loss of mass as carbon dioxide gas is produced during the reaction.
What does the graph of mass of reactants against time represent?
-The graph represents the decrease in mass of reactants over time. The slope (gradient) of the graph indicates the rate of the reaction at different time points.
What happens to the rate of reaction as it proceeds in the marble chips and hydrochloric acid reaction?
-As the reaction proceeds, the rate of reaction slows down, meaning the reaction rate decreases over time.
How can the rate of reaction be measured by tracking the products?
-The rate of reaction can also be measured by tracking the volume of carbon dioxide gas produced, using an apparatus designed to capture and measure gas volume over time.
What does the graph of volume of gas produced against time show?
-The graph shows the increase in volume of carbon dioxide gas over time. Similar to the reactant graph, the slope (gradient) at different time points indicates the rate of the reaction.
Why does the rate of reaction decrease over time?
-The rate of reaction decreases over time because, as the reaction progresses, fewer reactant molecules are available for collisions, which slows down the reaction.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
5.0 / 5 (0 votes)