DUNIA QUANTUM EMANG AGAK LAIN!! TEMBOK SAJA BISA TEMBUS!!
Summary
TLDRThe video explains the concept of energy in physics, focusing on how objects cannot overcome barriers without sufficient kinetic energy. It highlights quantum tunneling, a phenomenon where particles can pass through barriers even if their energy is lower than the barrier's potential energy. The video touches on atomic structure, electrostatic forces, and the Pauli exclusion principle, explaining why solid objects don't pass through each other. It also delves into how quantum tunneling powers nuclear fusion in the Sun and photosynthesis, demonstrating its vital role in sustaining life on Earth.
Takeaways
- 🌄 Energy must exceed potential barriers: Just as the character struggles to cross a valley, we need sufficient kinetic energy to overcome potential barriers.
- 🚀 Gravitational barriers: Humans cannot fly because our kinetic energy is not enough to overcome Earth's gravitational pull.
- ⚡ Electrostatic repulsion: The reason objects feel solid is due to electrostatic forces between atoms, which prevent them from touching each other.
- 🧲 Atoms repel: Much like magnets, atoms repel each other when their electrons come close, giving the illusion of solidity.
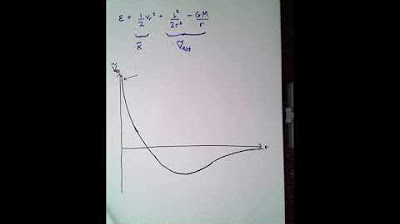
- 🌌 Quantum tunneling: In the quantum world, particles can sometimes pass through barriers, even with insufficient energy, known as Quantum Tunneling.
- ⚛️ Atomic structure: Atoms are mostly empty space, with extremely tiny particles (protons, neutrons, electrons) that make up everything, including our bodies.
- 🔒 Pauli exclusion principle: Electrons cannot occupy the same space or state, creating unique configurations that prevent atoms from overlapping.
- 💥 Sun’s energy: The sun shines because of quantum tunneling, enabling hydrogen atoms to fuse into helium in a nuclear reaction.
- 🌿 Photosynthesis and quantum mechanics: Quantum tunneling plays a role in photosynthesis, allowing light particles to enter chlorophyll and enable plant life.
- 🔭 Quantum weirdness: While quantum effects seem bizarre, they are essential to life processes, from the sun's energy to how we perceive the world as solid.
Q & A
What is the core concept presented in the video about energy in physics?
-The video explains that in physics, overcoming obstacles requires sufficient energy. If an object lacks enough kinetic energy, it can't overcome potential energy barriers, as demonstrated by an example of a person not being able to cross a valley without enough kinetic energy.
Why can't humans fly or pass through solid walls, according to classical physics?
-Humans can't fly because their kinetic energy isn't enough to overcome the gravitational field, which acts as an energy barrier. Similarly, walls can't be penetrated because their atoms create electrostatic forces that repel other atoms, making solid objects seem impenetrable.
What is quantum tunneling, and how does it differ from classical energy principles?
-Quantum tunneling is a phenomenon where particles can pass through energy barriers even if they lack the required energy. Unlike classical physics, where objects need sufficient energy to overcome obstacles, quantum particles have a probability of 'appearing' on the other side of barriers due to their wave-like nature.
How does the exclusion principle (Pauli's Exclusion Principle) affect atoms' behavior?
-The exclusion principle states that no two electrons can occupy the same quantum state within an atom. This prevents electrons from overlapping or occupying the same orbit, ensuring that atoms maintain specific configurations and don't simply merge together.
Why don't human bodies, made up of mostly empty space, pass through other objects?
-Though atoms are mostly empty space, the electrons surrounding them create electrostatic fields that repel other atoms. This repulsion is what gives the sensation of solidity, preventing human bodies from passing through objects.
What role does quantum tunneling play in the existence of life?
-Quantum tunneling is essential for life. It enables nuclear fusion in the Sun, allowing hydrogen atoms to overcome electrostatic repulsion and form helium. Without quantum tunneling, the Sun wouldn't produce the energy necessary for life. Similarly, it aids in photosynthesis by helping light particles (photons) enter plant cells.
Why is the probability of a human passing through a wall using quantum tunneling nearly impossible?
-The probability of a human passing through a wall using quantum tunneling is astronomically small. While a few particles may tunnel, the sheer number of particles in a human body makes the likelihood extremely low, making such an event practically impossible.
How do electrostatic forces between atoms prevent them from merging?
-Electrostatic forces, specifically the repulsion between negatively charged electrons, prevent atoms from merging. These forces act similarly to how like poles of magnets repel each other, ensuring that atoms don't touch or overlap.
What does the video suggest about the nature of electrons in quantum mechanics?
-In quantum mechanics, electrons are described not as physical objects but as probability waves. This means they don't have fixed positions and could be located in various places at once, described by a probability distribution known as a wave function.
Why is the phenomenon of quantum tunneling important for both nuclear fusion and photosynthesis?
-Quantum tunneling allows particles in the Sun to overcome energy barriers, enabling nuclear fusion, which powers the Sun and provides energy to Earth. It also plays a critical role in photosynthesis, as light particles tunnel into plant cells to initiate the process of converting sunlight into chemical energy.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
5.0 / 5 (0 votes)