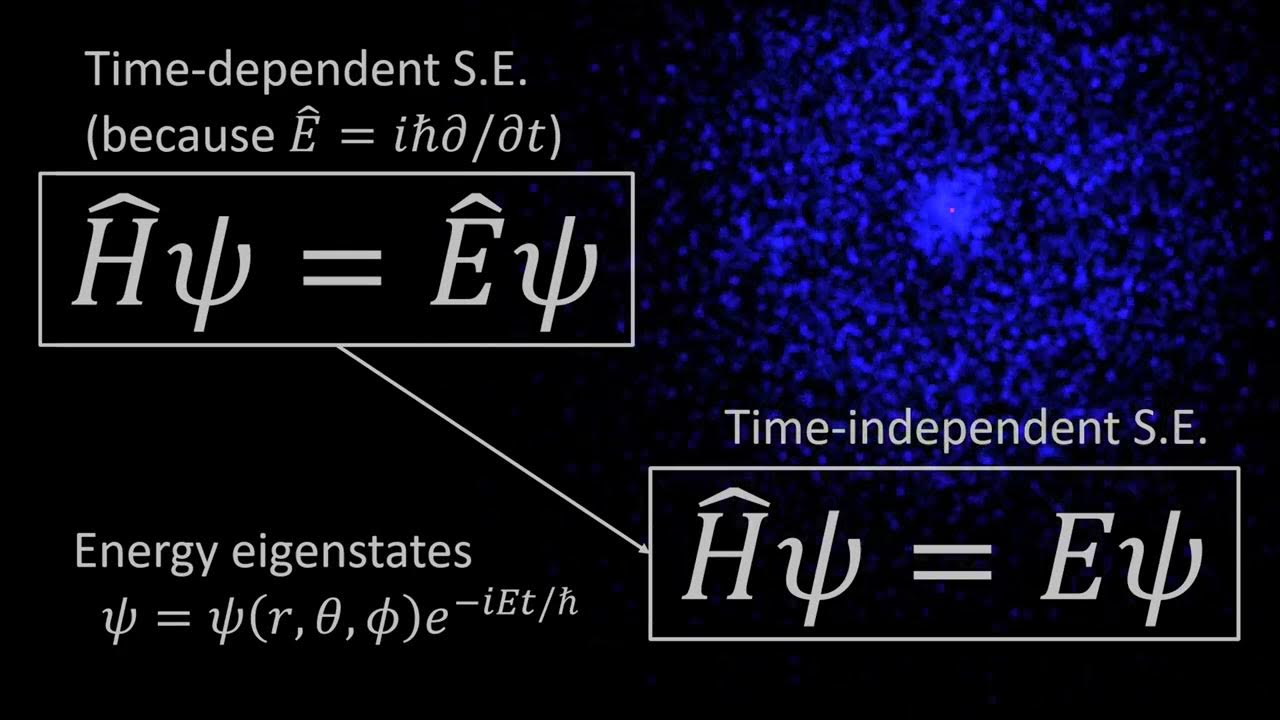
The spherical harmonics
Summary
TLDRIn this video, Professor Erin Das explores spherical harmonics, essential eigenfunctions in quantum mechanics, particularly relevant to the hydrogen atom. The discussion covers their mathematical forms, visualization techniques, and their role in describing orbital angular momentum. Using visual representations on the unit sphere, the video explains the angular dependence of spherical harmonics and how they vary with quantum numbers l and m. The content is supported by Python code available through a linked Jupyter notebook, encouraging viewers to interact with and generate their own visualizations. This video is ideal for anyone interested in the mathematical beauty and application of spherical harmonics in quantum systems.
Takeaways
- 📚 Spherical harmonics are the eigenfunctions of orbital angular momentum in quantum mechanics, crucial in many problems, especially in the hydrogen atom.
- 🔢 These harmonics depend on two quantum numbers: l (angular momentum) and m (magnetic quantum number).
- 📈 Spherical harmonics are often visualized on the unit sphere, where positive values are red, negative values are blue, and zero values are white.
- 🧮 The Y00 spherical harmonic is a constant function, representing a uniform red sphere, while Ylm functions show more complex patterns depending on l and m.
- 🎨 The visualization of spherical harmonics helps in understanding their angular dependence, with each harmonic represented by color-coded spheres based on their values.
- 🔄 The values of spherical harmonics can change with the angles θ and φ, producing varying patterns across different harmonics as l and m increase.
- 💻 The script refers to a linked Jupyter notebook containing Python code that can generate spherical harmonics, allowing viewers to visualize them themselves.
- 🔬 Spherical harmonics play a central role in quantum mechanics, especially in understanding the angular part of wavefunctions in the hydrogen atom.
- 🔍 The script also explains how spherical harmonics are related to associated Legendre polynomials and their use in different mathematical forms.
- 🔧 An alternative way to visualize spherical harmonics is by plotting the magnitude of the function, which produces different, often used, visual representations.
Q & A
What are spherical harmonics and why are they important in quantum mechanics?
-Spherical harmonics are the eigenfunctions of orbital angular momentum in quantum mechanics. They are important because they describe the angular part of wavefunctions in systems such as the hydrogen atom, making them crucial for solving problems involving spherical symmetry.
What is the general form of a spherical harmonic function?
-The general form of a spherical harmonic function \( Y_{l}^{m}(θ, φ) \) consists of a pre-factor, a phase factor, and a term involving associated Legendre polynomials, depending on the angular variables \( θ \) (colatitude) and \( φ \) (azimuthal angle).
What are the quantum numbers associated with spherical harmonics?
-Spherical harmonics are labeled by two quantum numbers: \( l \), which is associated with the magnitude of orbital angular momentum, and \( m \), which describes the z-component of angular momentum. The values of \( l \) are non-negative integers, and for a given \( l \), \( m \) ranges from \( -l \) to \( l \).
How do spherical harmonics relate to the hydrogen atom?
-In the hydrogen atom, spherical harmonics describe the angular part of the electron's wavefunction. This is due to the spherical symmetry of the atom, making spherical harmonics essential for solving the Schrödinger equation in spherical coordinates.
What do the colors in spherical harmonic visualizations represent?
-In spherical harmonic visualizations, colors represent the value of the function at each angular position on the unit sphere. Red indicates positive values, blue indicates negative values, and white corresponds to zero. These visualizations help show the angular dependence of the function.
How are spherical coordinates used in the context of spherical harmonics?
-Spherical coordinates are used to describe the position of a point in space using three numbers: \( r \) (distance from the origin), \( θ \) (angle from the z-axis), and \( φ \) (angle in the horizontal plane from the x-axis). Spherical harmonics depend only on \( θ \) and \( φ \), making them ideal for problems with spherical symmetry.
What are associated Legendre polynomials and their role in spherical harmonics?
-Associated Legendre polynomials, denoted as \( P_{l}^{m}(\cos θ) \), appear in the mathematical expression of spherical harmonics. They define the angular dependence of spherical harmonics in terms of the colatitude angle \( θ \).
What does the spherical harmonic \( Y_{0}^{0} \) look like?
-The spherical harmonic \( Y_{0}^{0} \) is a constant and has no angular dependence. It is represented by a uniformly red sphere because the function has the same value at all points on the unit sphere, corresponding to a value of \( 1 / \sqrt{4π} \).
How do the real and imaginary parts of spherical harmonics differ in their visual representation?
-The real and imaginary parts of spherical harmonics can be represented separately. For example, for \( Y_{1}^{-1} \), the real part exhibits a cosine dependence, while the imaginary part exhibits a sine dependence. These two parts are 90 degrees out of phase and show different patterns in the color plot.
What are the common methods for visualizing spherical harmonics, and how do they differ?
-There are two common methods for visualizing spherical harmonics. One plots the function on a unit sphere, using colors to represent the value at different angles. Another method uses the magnitude of the function to define a radial distance, effectively stretching the surface based on the function's value. Both methods highlight different aspects of the function's angular dependence.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
5.0 / 5 (0 votes)