4.1 Development of a New Atomic Model
Summary
TLDRThis lecture discusses the development of a new atomic model, addressing the limitations of Rutherford's model in explaining the behavior of the nucleus and electrons. It introduces the dual nature of light, behaving as both a wave and a particle, and explores the photoelectric effect, where light interacts with metal to emit electrons. Key concepts include Max Planck’s quantum theory, the relationship between energy and frequency, and Einstein’s photon theory. The lecture also introduces Bohr's model of the atom, focusing on electron orbits, energy states, and the hydrogen atom’s line emission spectrum.
Takeaways
- 🔬 Rutherford's atomic model was incomplete as it didn't explain why the nucleus didn't fly apart despite having positive charges.
- ⚛️ The model also couldn't explain why negatively charged electrons didn't fall into the positively charged nucleus.
- 💡 The development of a new atomic model began with experiments involving light, revealing its wave-particle duality.
- 🌈 Light is a form of electromagnetic radiation, and only a small portion of the electromagnetic spectrum is visible to the human eye.
- 🌊 Two key properties of light waves are wavelength (Lambda) and frequency, which are inversely related to maintain the constant speed of light (C = 3 x 10^8 m/s).
- 📡 The photoelectric effect demonstrated that light must reach a certain frequency before electrons are emitted from a metal surface.
- 📏 Max Planck proposed that energy is emitted in discrete packets called quanta, relating energy to frequency with the formula E = hf.
- ✨ Einstein further developed this by describing light as photons, showing that atoms absorb whole photons, not fractions.
- 🔬 The hydrogen atom's emission spectrum showed that excited atoms release specific wavelengths of light when returning to their ground state.
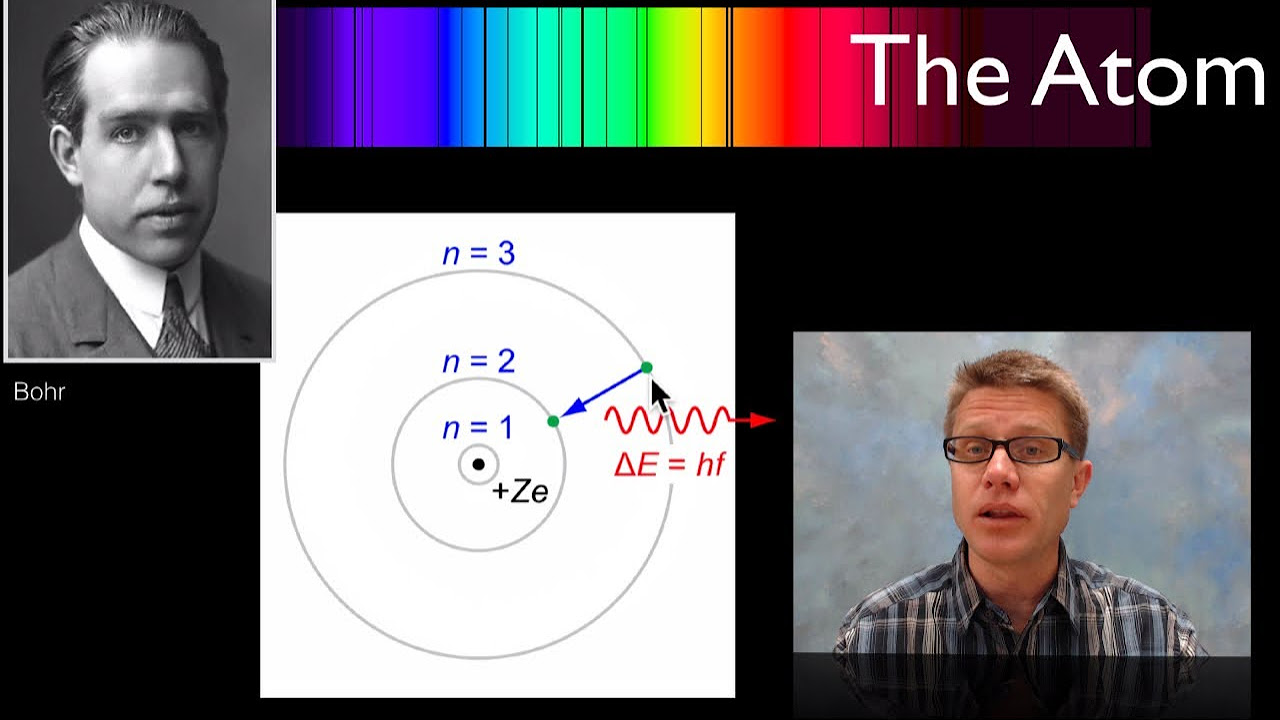
- 🔭 Niels Bohr proposed a model where electrons move in stable orbits, and only certain energy levels are allowed, explaining why hydrogen emitted discrete spectral lines.
Q & A
Why did scientists need to develop a new atomic model?
-Scientists needed a new atomic model because Rutherford's model was incomplete. It didn't explain why the positively charged nucleus didn't fly apart due to the repulsion of like charges, nor did it explain why negatively charged electrons didn't fall into the nucleus due to their attraction to the positive charge.
What is the significance of white light in the development of the atomic model?
-White light is significant because it led to the understanding that light behaves as both a particle and a wave. This dual nature helped scientists develop new ideas about atomic structure and the behavior of electrons.
What is the electromagnetic spectrum and how does it relate to light?
-The electromagnetic spectrum is a range of all possible frequencies of electromagnetic radiation, including radio waves, visible light, X-rays, and gamma rays. The light we see is a small part of this spectrum, and understanding its properties helped scientists understand atomic behavior.
What are the two basic properties of waves mentioned in the script?
-The two basic properties of waves mentioned are wavelength (Lambda), which is the distance between two crests or troughs in the wave, and frequency, which is how many crests pass by every second.
How is the speed of light related to wavelength and frequency?
-The speed of light (C) is related to wavelength (Lambda) and frequency (F) by the equation C = Lambda * F. This equation shows that wavelength and frequency are inversely related, meaning if one increases, the other must decrease to maintain the constant speed of light.
What is the photoelectric effect and how does it challenge the continuous wave theory of light?
-The photoelectric effect is the emission of electrons from a material when light shines upon it. It challenges the continuous wave theory because electrons are only emitted at a certain minimum frequency, and not at lower frequencies regardless of light intensity, suggesting that light must be quantized rather than continuous.
Who proposed the idea that light is emitted in packets called quanta, and how did this relate to the photoelectric effect?
-Max Planck proposed the idea that light is emitted in packets called quanta. This concept related to the photoelectric effect by explaining that only certain frequencies (and thus certain quanta of energy) could cause electrons to be emitted from a material.
What is Planck's constant and how does it relate to the energy of quanta?
-Planck's constant (H) is a fundamental physical constant that relates the energy (E) of a quantum to its frequency (F) by the equation E = H * F. It is approximately 6.626 x 10^-34 joule seconds and is crucial for understanding the energy carried by photons.
How did Einstein's explanation of the photoelectric effect build upon Planck's idea of quanta?
-Einstein explained that light consists of particles called photons and that atoms and molecules can only absorb whole numbers of photons. This meant that there was a minimum energy required to eject an electron, which explained the observed threshold in the photoelectric effect and built upon Planck's quantized concept of energy.
What are energy states in the context of atomic physics?
-Energy states are levels of energy that an atom can have, which include both kinetic and potential energy. The ground state is the lowest energy state, and higher states are called excited states.
How did the study of hydrogen's line emission spectrum contribute to atomic theory?
-The study of hydrogen's line emission spectrum showed that light was emitted at specific wavelengths when hydrogen atoms returned to their ground state from an excited state. This indicated that atoms have quantized energy levels and that electrons can only occupy certain stable orbits, which was a key part of the development of quantum theory.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade Now5.0 / 5 (0 votes)