Acid Base Physiology | Part Three | Renal Regulation | Acidification of Urine | Renal Physiology
Summary
TLDRThis Bite Size Med video delves into the kidney's role in acid-base balance regulation. It explains the Henderson-Hasselbalch equation, the importance of bicarbonate and carbon dioxide as the main buffer pair, and how kidneys respond to changes in pH by adjusting hydrogen ion secretion and bicarbonate reabsorption. The video also covers the nephron's function in bicarbonate reabsorption and hydrogen ion secretion, the role of urinary buffers like phosphate and ammonia, and how kidneys compensate for respiratory and metabolic acid-base disturbances.
Takeaways
- 🧠 The kidneys play a crucial role in regulating acid-base balance in the body alongside buffers and the lungs.
- 🔍 The Henderson-Hasselbalch equation (pH = pK + log [base]/[acid]) is fundamental in understanding pH regulation, where changes in hydrogen ion concentration affect pH levels.
- 🌡 A normal blood pH is around 7.4, indicating a neutral to slightly alkaline state.
- 🔄 The bicarbonate-carbon dioxide buffer pair is the most significant in maintaining pH balance, with the kidneys regulating bicarbonate levels.
- 💧 The kidneys respond slowly but strongly to changes in hydrogen ion concentrations, managing high or low bicarbonate levels by adjusting hydrogen ion secretion and bicarbonate production.
- 🔁 Bicarbonate reabsorption primarily occurs in the early part of the nephron, where hydrogen ions are secreted, and bicarbonate is reabsorbed through secondary active transport.
- ⏳ Hydrogen ion secretion mainly takes place in the late distal convoluted tubule and collecting duct, involving primary active transport mechanisms.
- 🧪 The kidneys use urinary buffers, such as phosphate and ammonia buffers, to manage the excretion of hydrogen ions and maintain acid-base balance.
- 🔄 In response to respiratory acidosis, the kidneys reabsorb more bicarbonate and excrete more hydrogen ions to compensate for the increased CO2 levels.
- 🔄 Conversely, in respiratory alkalosis, the kidneys reduce bicarbonate reabsorption and hydrogen ion excretion to counteract decreased CO2 levels.
- 🔄 The kidneys can compensate for all four primary acid-base disturbances, demonstrating their critical role in the body's acid-base homeostasis.
Q & A
What are the three regulatory systems of acid-base balance mentioned in the script?
-The three regulatory systems of acid-base balance are buffers, the lungs, and the kidneys.
What does the Henderson-Hasselbach equation represent?
-The Henderson-Hasselbach equation represents the pH of a solution, which is calculated as pH = pK + log([base]/[acid]).
What is the normal pH of blood, and what does a change in pH indicate?
-The normal pH of blood is around 7.4. An increase in hydrogen ion concentration reduces the pH, indicating acidosis, while a decrease in hydrogen ion concentration raises the pH, indicating alkalosis.
What is the most important buffer pair in the body, and what is its pK value?
-The most important buffer pair in the body is the bicarbonate (HCO3-) and carbon dioxide (CO2) pair, with a pK of 6.1.
How do kidneys regulate bicarbonate concentration?
-Kidneys regulate bicarbonate concentration by excreting more hydrogen ions and producing new bicarbonate when there are high hydrogen ion concentrations and low bicarbonate, and by reducing hydrogen ion secretion and excreting more bicarbonate when there are low hydrogen ions and high bicarbonate.
In which parts of the nephron does bicarbonate reabsorption and hydrogen ion secretion primarily occur?
-Bicarbonate reabsorption primarily occurs in the early part of the proximal convoluted tubule (PCT), while hydrogen ion secretion occurs more in the late distal convoluted tubule (DCT) and collecting duct.
How does the sodium-hydrogen exchanger contribute to bicarbonate reabsorption?
-The sodium-hydrogen exchanger contributes to bicarbonate reabsorption by using the energy from the sodium-potassium ATPase to exchange sodium for hydrogen ions, which helps drive the reabsorption of bicarbonate.
What is the role of carbonic anhydrase in the nephron?
-Carbonic anhydrase in the nephron plays a role in catalyzing the conversion of carbon dioxide and water into carbonic acid, which then dissociates into hydrogen ions and bicarbonate ions, facilitating the secretion of hydrogen ions and reabsorption of bicarbonate.
What are the two urinary buffers mentioned in the script, and how do they help in acid-base regulation?
-The two urinary buffers mentioned are the phosphate buffers and the ammonia buffers. They help in acid-base regulation by binding with hydrogen ions to form titratable acids, which can be excreted, thus helping to maintain acid-base balance.
How does the kidney compensate for respiratory acidosis and alkalosis?
-In respiratory acidosis, where the partial pressure of carbon dioxide (PCO2) is high, the kidneys compensate by reabsorbing more bicarbonate and excreting more hydrogen ions. In respiratory alkalosis, where PCO2 is low, the kidneys reduce bicarbonate reabsorption and hydrogen ion excretion.
What happens in the kidney during metabolic acidosis and alkalosis?
-During metabolic acidosis, where blood hydrogen ion levels are high and bicarbonate is low, the kidney removes excess hydrogen ions and retains more bicarbonate. In metabolic alkalosis, the kidney does the opposite, reducing hydrogen ion secretion and bicarbonate reabsorption.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Respiratory Response To Exercise | Respiratory Physiology

CO2 Transport | Carbon-di-oxide Transport | Respiratory Gas Exchange | Respiratory Physiology
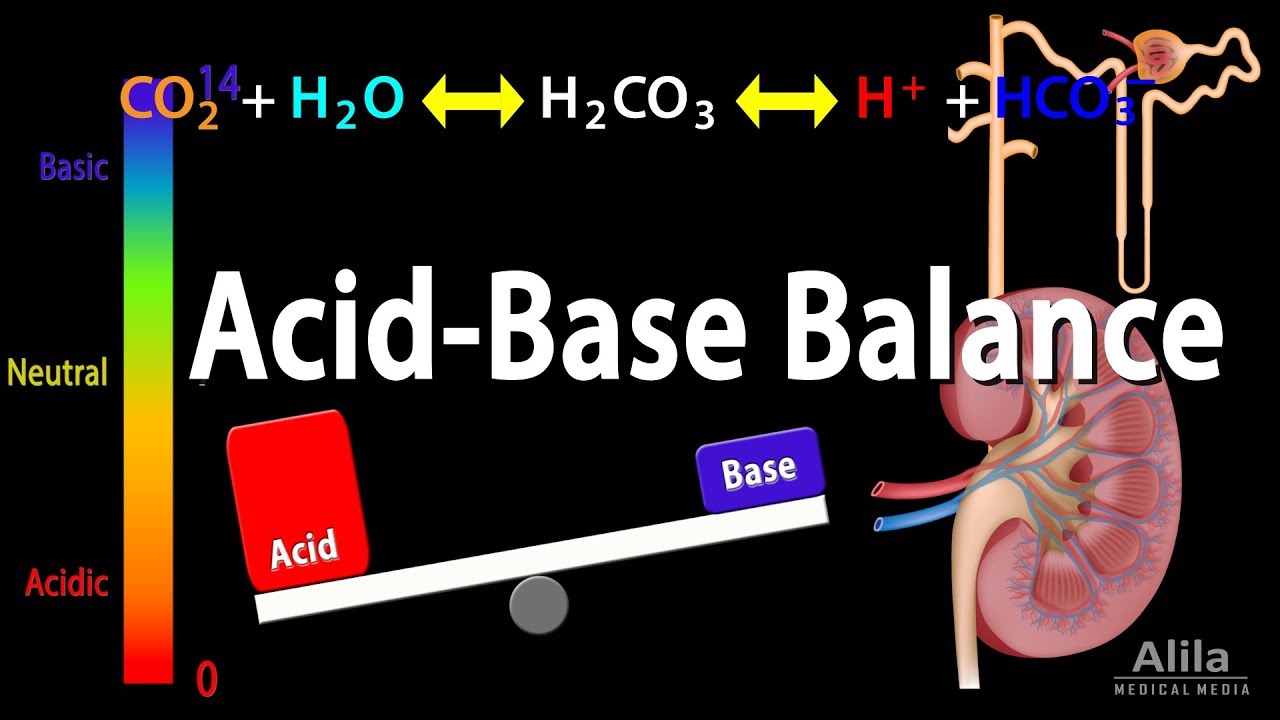
Acid Base Balance, Animation.

Nephron

20201 - PSF107 - ANATOMI DAN FISIOLOGI MANUSIA - 1 (TYAS PUTRI UTAMI) ***

Stoikiometri Larutan • Part 2: Persamaan Ion Kelompok Reaksi Asam-Basa
5.0 / 5 (0 votes)