Fischer projection introduction | Stereochemistry | Organic chemistry | Khan Academy
Summary
TLDRThe video script explains the use of Fischer projections to represent molecules, particularly for chirality centers. It details how to assign configurations to chiral centers using priority rules and visual tricks, emphasizing the importance of hydrogen's position. The instructor demonstrates two methods for determining R or S configurations and shows how to draw the enantiomer of a compound using a mirror image technique, highlighting the reversal of configurations at chiral centers.
Takeaways
- 🔬 Fischer projections are a method for representing molecules, particularly useful for depicting chirality centers.
- 🏅 Emil Fischer, the creator of Fischer projections, won the Nobel Prize in Chemistry for his work on carbohydrates.
- 🧬 A chirality center in a molecule is a carbon atom bonded to four different groups, which can lead to different spatial configurations.
- 🌐 In Fischer projections, horizontal lines represent bonds coming out of the page, which are depicted as wedges in three-dimensional drawings.
- 🔽 Vertical lines in Fischer projections indicate bonds going into the page, represented as dashes in three-dimensional drawings.
- 📉 Assigning configuration to a chirality center involves determining the spatial arrangement of the four attached groups, with hydrogen typically having the lowest priority.
- 🔄 The process of assigning R or S configuration to a chirality center involves visualizing the molecule from a specific perspective and following the clockwise or counterclockwise direction of the highest priority groups.
- 🔄 A second method for assigning configuration involves ignoring the hydrogen and determining the direction (clockwise or counterclockwise) of the remaining highest priority groups.
- 🔍 A model set can be a helpful tool for visualizing and practicing the assignment of configurations to chirality centers.
- 🪞 To draw the enantiomer of a compound, the mirror method can be used, reflecting the molecule across a hypothetical mirror plane to reverse the configuration at each chirality center.
Q & A
What is a Fischer projection?
-A Fischer projection is a method of representing the structure of chiral molecules, particularly carbohydrates, in two dimensions. It was developed by Emil Fischer and is used to depict the spatial arrangement of atoms around a chirality center.
Why is the carbon atom in the Fischer projection considered a chirality center?
-The carbon atom is considered a chirality center because it is bonded to four different groups, which gives it a tetrahedral geometry and the ability to exist in non-superimposable mirror-image configurations known as enantiomers.
What do the horizontal and vertical lines in a Fischer projection represent?
-In a Fischer projection, a horizontal line represents a bond that is coming out of the page towards the viewer, while a vertical line represents a bond that is going away from the viewer into the page.
How is the spatial orientation of bonds represented in a Fischer projection?
-The spatial orientation of bonds in a Fischer projection is represented by using wedges for bonds coming out of the page and dashes for bonds going into the page, with the carbon atom at the center of the projection.
What is the significance of assigning priorities to the groups attached to a chirality center?
-Assigning priorities to the groups attached to a chirality center is crucial for determining the absolute configuration of the molecule. This is done by ranking the groups based on their atomic numbers and using the Cahn-Ingold-Prelog priority rules.
How does one determine the R or S configuration of a chirality center from a Fischer projection?
-The R or S configuration is determined by visualizing the molecule from a perspective where the lowest priority group (usually hydrogen) is pointing away from the viewer. The direction of the remaining groups (from highest to lowest priority) is then followed to decide if the configuration is clockwise (R) or counterclockwise (S).
What is the trick mentioned in the script for determining the R or S configuration when the hydrogen is coming out at you in a Fischer projection?
-The trick is to ignore the hydrogen and look at the other three groups. If the direction of the groups from highest to lowest priority appears counterclockwise, the actual configuration is R, and if it appears clockwise, it is S.
How can one draw the enantiomer of a compound using a Fischer projection?
-To draw the enantiomer of a compound, one can use the mirror method. This involves reflecting the compound across a mirror plane, reversing the spatial orientation of all groups, and ensuring that the configuration at each chirality center is inverted.
Why is it important to use a model set when learning to assign configurations to chirality centers?
-Using a model set helps in visualizing the three-dimensional structure of the molecule and understanding the spatial relationships between the groups attached to the chirality center. This hands-on approach can make it easier to grasp the concept and apply it to different molecules.
What is the difference between a wedge and a dash in a Fischer projection?
-In a Fischer projection, a wedge represents a bond that is coming out of the page towards the viewer, while a dash represents a bond that is going into the page away from the viewer. These notations help to indicate the stereochemistry of the molecule.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Enantiomers
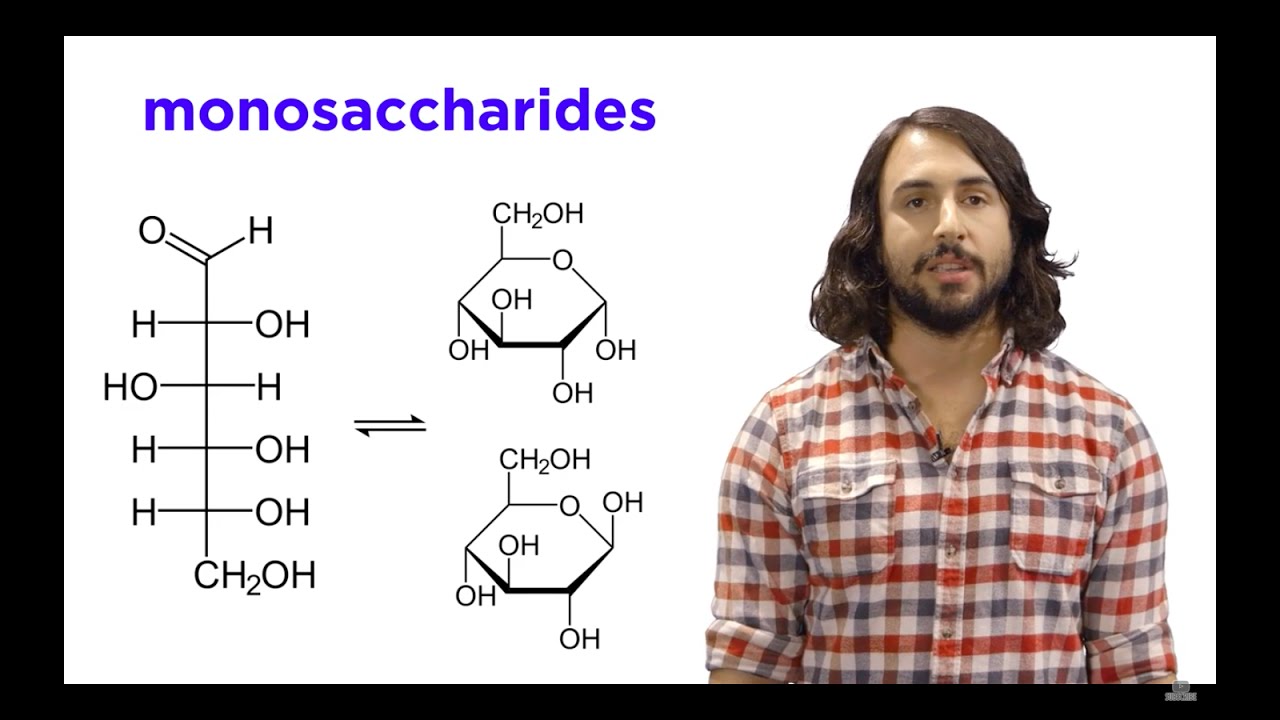
Carbohydrates Part 1: Simple Sugars and Fischer Projections

Arkeolog Pakai Senyawa Ini | Diastereomer | Stereokimia 3 | Kuliah Online | Kimia Organik

Meso compounds | Stereochemistry | Organic chemistry | Khan Academy

Stereochemistry: Meso Compounds, Diastereomers

Stereochemistry: Crash Course Organic Chemistry #8
5.0 / 5 (0 votes)