The periodic table | Atoms, elements, and the periodic table | High school chemistry | Khan Academy
Summary
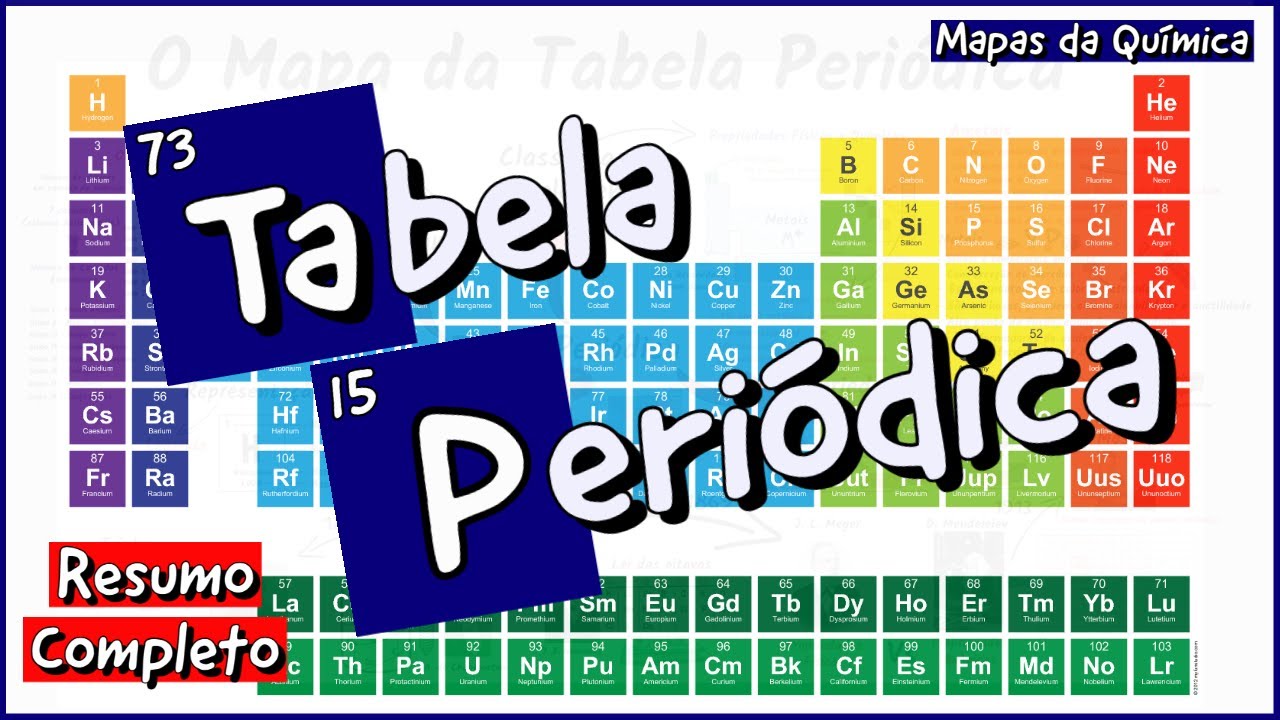
TLDRThis educational video script explores the periodic table's organization, focusing on groups and periods. It explains how elements are classified into groups (vertical columns) and periods (horizontal rows), with groups numbered 1 to 18 and periods 1 to 6. The script highlights alkali metals in group 1A, alkaline earth metals in group 2A, and discusses their reactivity and states in nature. It contrasts metals, typically found on the left side, with nonmetals on the right, and introduces metalloids with properties between the two. The video also touches on halogens and noble gases, setting the stage for further discussions on electronic structure and transition metals.
Takeaways
- 🔍 The periodic table organizes elements into vertical columns called groups, numbered from 1 to 18.
- 📏 Another way to label groups is using A and B categories, such as 1A, 2A, and skipping to 3A for group 13.
- 🔬 The concept of periods refers to the horizontal rows on the periodic table, with elements like hydrogen and helium in the first period.
- 🟥 Alkali metals, found in group 1 or 1A, are soft, silvery, and extremely reactive metals that are never found in their pure state in nature.
- 🟩 Hydrogen, despite being in group 1, is a nonmetal and does not share the same reactivity as alkali metals.
- 🟨 Alkaline earth metals, located in group 2 or 2A, are less reactive than alkali metals but are also found in combination with other elements in nature.
- 🛠 Metals, in general, are malleable and ductile, and they conduct heat and electricity well, with the exception of mercury which is liquid at room temperature.
- 🟦 Nonmetals are typically poor conductors of heat and electricity, and solid nonmetals are brittle rather than malleable.
- 🌐 Halogens, found in group 7A or 17, are very reactive nonmetals known for their corrosive nature and ability to form salts.
- 💨 Noble gases, located in group 8A or 18, are colorless, unreactive gases with complete electron shells making them stable.
- 📏 The periodic table is divided into metals on the left, nonmetals on the right, and metalloids along a zigzag line in between, which have properties of both.
Q & A
What are the groups on the periodic table?
-The groups on the periodic table are the vertical columns, numbered from 1 to 18. They are also referred to as groups 1A to 8A, excluding groups 3 through 12.
How are the groups numbered on the periodic table?
-Groups on the periodic table can be numbered in two ways: either by simply numbering them 1 to 18, or by using the A system where groups 1 and 2 are called 1A and 2A, and groups 13 to 18 are labeled 3A to 8A.
What is the significance of valence electrons in group numbering?
-The second way of numbering groups, using the A system, is useful when considering valence electrons because it helps in understanding the chemical properties and reactivity of elements within the same group.
What is a period on the periodic table?
-A period on the periodic table is a horizontal row, with the first period containing hydrogen and helium, and each subsequent period adding more elements.
Why are alkali metals found in group 1 or 1A?
-Alkali metals are found in group 1 or 1A because they all have one electron in their outermost shell, which they readily lose to form positive ions, making them highly reactive.
How do alkali metals react with water?
-Alkali metals react vigorously with water, often producing a hydrogen gas and an alkaline solution, due to their high reactivity and tendency to lose their single valence electron.
Why are alkali metals not found in their pure state in nature?
-Alkali metals are not found in their pure state in nature because of their extreme reactivity. They are typically found combined with other elements in compounds.
What distinguishes hydrogen from other alkali metals?
-Hydrogen is in group 1 but is not an alkali metal; it is a nonmetal. This distinction is due to its unique properties and electron configuration, which do not align with the typical characteristics of alkali metals.
What are alkaline earth metals and where are they located on the periodic table?
-Alkaline earth metals are found in group 2 or 2A and include elements like magnesium, calcium, and strontium. They are less reactive than alkali metals but share similar chemical properties.
What are the general properties of metals?
-Metals are typically solid at room temperature, malleable, ductile, and good conductors of heat and electricity. They are found on the left side of the periodic table.
How are nonmetals different from metals in terms of properties?
-Nonmetals are generally poor conductors of heat and electricity, can be brittle, and are found in various states of matter. They are located on the right side of the periodic table.
What are halogens and where can they be found on the periodic table?
-Halogens are very reactive nonmetals found in group 7A or 17. They include elements like fluorine, chlorine, and bromine, and are known for their corrosive properties and tendency to form salts.
Why are noble gases unreactive?
-Noble gases are unreactive because they have a full valence shell of electrons, making them stable and less likely to form chemical bonds with other elements.
What is the dividing line between metals and nonmetals on the periodic table?
-The dividing line between metals and nonmetals on the periodic table is a zigzag line that separates the elements with metallic properties on the left from those with nonmetallic properties on the right.
What are metalloids and which elements are commonly considered metalloids?
-Metalloids are elements with properties intermediate between metals and nonmetals. Commonly considered metalloids include boron, silicon, germanium, arsenic, antimony, tellurium, and sometimes astatine.
Why is silicon an important metalloid?
-Silicon is an important metalloid because it is a semiconductor, which means it can conduct electricity under certain conditions but not as readily as a metal, making it crucial in electronic devices.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
5.0 / 5 (0 votes)