Acids, Bases and Salts in 20 Minutes🔥| Class 10th | Rapid Revision | Prashant Kirad
Summary
TLDRThe script is a lecture on acids and bases, focusing on their definitions, properties, and reactions. It covers the release of H+ ions by acids and OH- ions by bases, and uses examples like lemons and blue litmus paper to explain concepts. The lecture also discusses natural acids like citric acid and indicators that help identify acids and bases. It further delves into reactions involving acids and bases, such as those with metals and metal oxides, leading to the formation of salts and water. The importance of pH in understanding the strength of acids and bases is highlighted, along with practical applications in everyday life, like in digestion, soil, and dental care.
Takeaways
- 📚 The next chapter to be revised is Acid Base and Salts, which is an important chapter for the exam.
- ⏰ The entire chapter will be revised quickly within the next 15 to 20 minutes, highlighting the top topics to focus on for the exam.
- 🔍 A detailed video around an hour and a half long is already available, covering the entire chapter in detail for those who want to understand the concepts deeply.
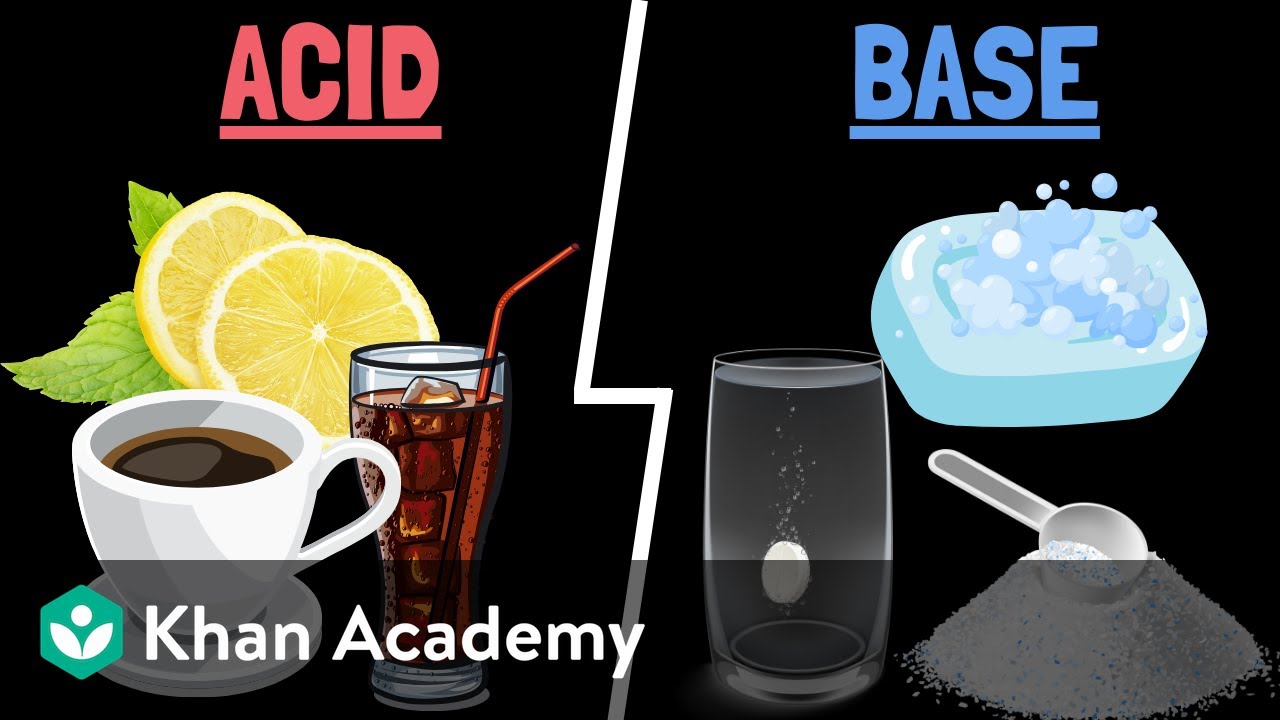
- 🌟 An important distinction is made between acids, which release hydrogen ions (H+) when dissolved in water, and bases, which release hydroxide ions (OH-).
- 🍋 Examples of natural acids are given, such as acetic acid in vinegar, citric acid in oranges, and ascorbic acid in tomatoes.
- 📊 A table is emphasized as very important, which will likely be referenced in an upcoming exam question, listing various natural acids found in different foods.
- 🌈 Natural indicators like litmus paper and their color changes with acids and bases are discussed, as well as synthetic indicators like phenolphthalein and methyl orange.
- 🔬 Reactions involving acids and bases, such as those with metals, metal carbonates, and metal oxides, are explained, including the concept of neutralization reactions.
- 💧 The concept of acid and base strength is introduced, relating to their ability to donate H+ ions and OH- ions, respectively, in water.
- 📈 pH is discussed as a measure of how acidic or basic a solution is, with examples of how it relates to digestion in the stomach, soil for plants, and tooth health.
- 🧂 The importance of salt formation when acids and bases react is highlighted, with examples of neutral, acidic, and basic salts depending on the strength of the reactants.
Q & A
What is the main topic of the chapter discussed in the script?
-The main topic of the chapter is 'Acids, Bases, and Salts', which is an important chapter in the context of the discussion.
What is the definition of an acid according to the script?
-An acid is defined as a substance that produces hydrogen ions (H+) when dissolved in water.
What is the difference between acids and bases as explained in the script?
-Acids are substances that produce hydrogen ions (H+) when dissolved in water, while bases produce hydroxide ions (OH-).
What is the role of litmus paper in the script's discussion of acids and bases?
-Litmus paper is used as a natural indicator to test for the presence of acids or bases. It turns red in the presence of an acid and blue in the presence of a base.
What are the examples of natural acids and bases mentioned in the script?
-Natural acids mentioned include acetic acid in vinegar, citric acid in oranges, and oxalic acid in tomatoes. A base example is sodium hydroxide, which is the main component of lye.
How does the script describe the reaction between acids and metals?
-The script explains that when an acid reacts with a metal, it typically produces a salt and hydrogen gas. However, nitric acid is an exception as it does not release hydrogen gas when reacting with metals like magnesium and manganese.
What is the significance of pH levels in the context of the script?
-The script emphasizes the importance of pH levels in understanding the acidity or alkalinity of a solution, with pH less than 7 indicating acidity and pH greater than 7 indicating alkalinity.
What is the purpose of discussing neutralization reactions in the script?
-Neutralization reactions are discussed to explain the process where an acid and a base react to form salt and water, which is a fundamental concept in chemistry.
How does the script connect the concept of acids and bases to everyday life?
-The script connects the concepts to everyday life by mentioning the use of baking soda (a base) in cooking and the role of stomach acid (a base) in digestion.
What is the significance of the 'chlor-alkali process' mentioned in the script?
-The chlor-alkali process is significant because it is an industrial method for producing sodium hydroxide, chlorine, and hydrogen, which are important chemicals used in various applications.
Why is the script's discussion on the reaction of acids with metal oxides important?
-The discussion on the reaction of acids with metal oxides is important because it illustrates how acids can be used to extract metals or produce salts and water, which has implications in metal processing and chemical synthesis.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

IPA kelas 9 BAB 5 reaksi kimia dan dinamikanya kurikulum merdeka asam dan basa

Larutan Asam, Basa, dan Garam (2021)|Materi IPA SMP Kelas 7|

The strengths and weaknesses of acids and bases - George Zaidan and Charles Morton
Intro to acids and bases | Chemistry | Khan Academy

Ácidos e bases de Lewis!!

Acids, Bases and Salts | Full Chapter | Class 10
5.0 / 5 (0 votes)