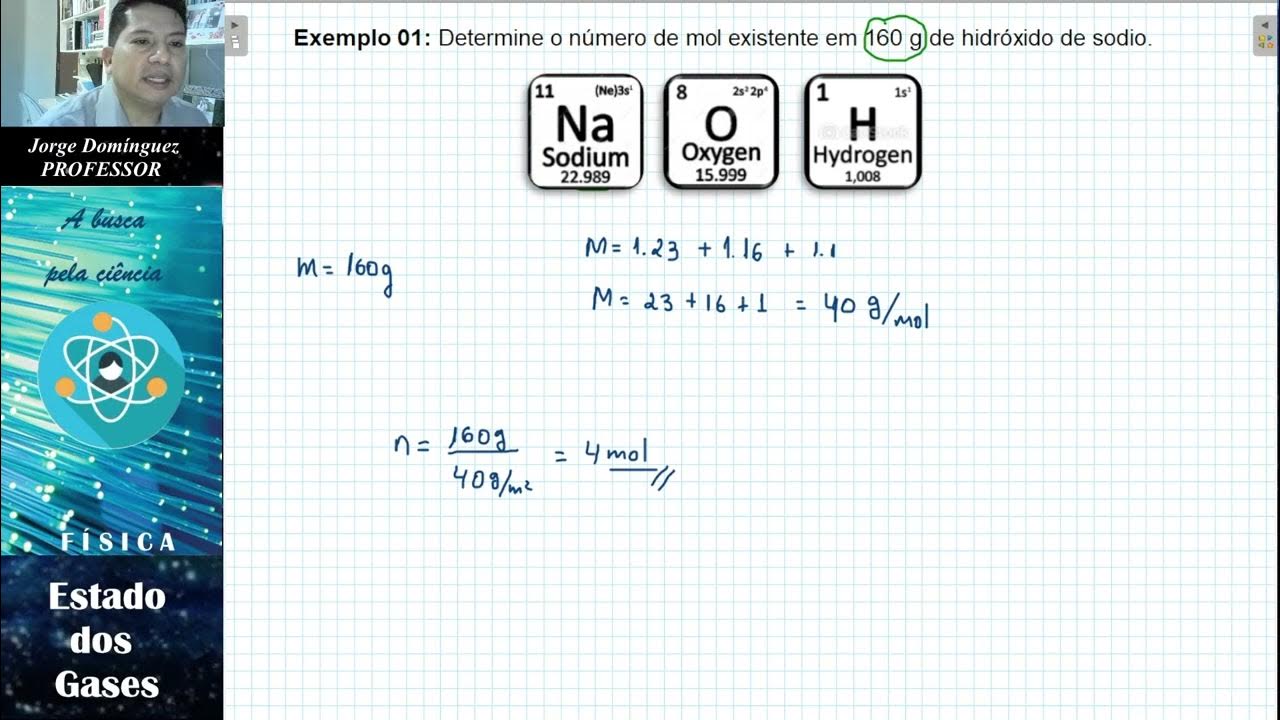
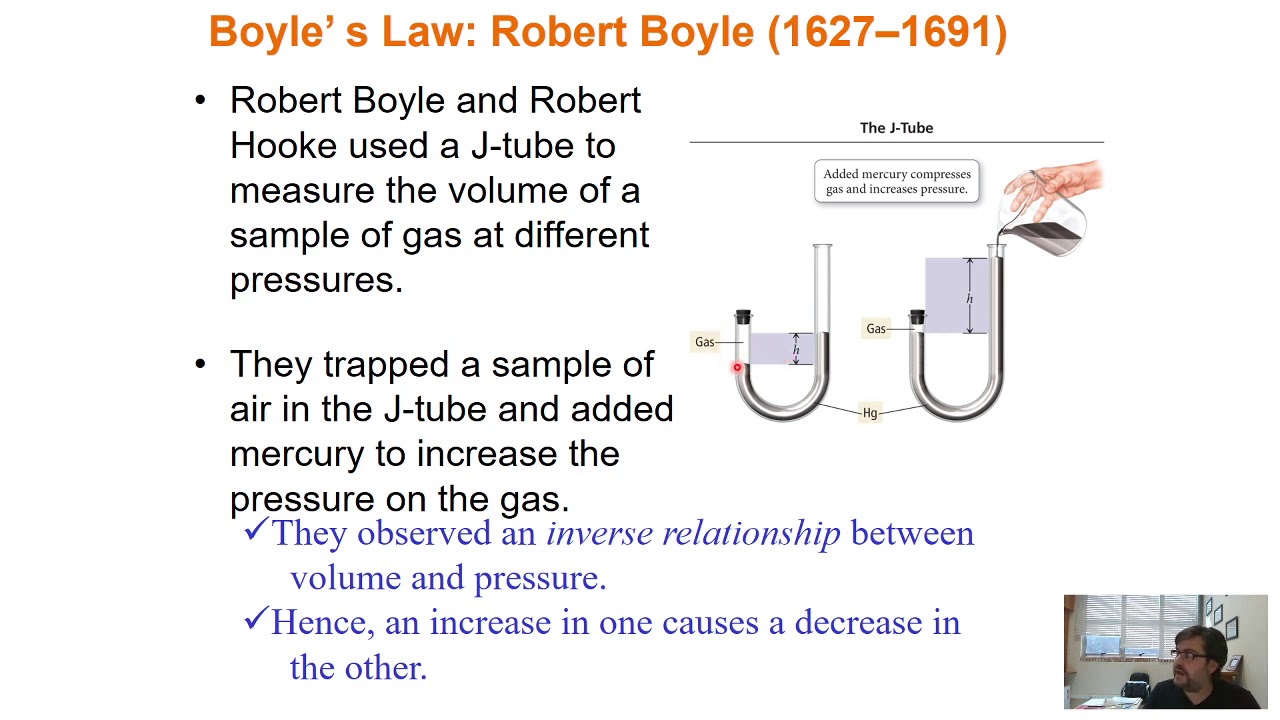
Thermodynamics- Maxwell-Boltzmann Law of Distribution- 1
Summary
TLDRThe video script discusses the molecular dynamics of gases, focusing on the importance of temperature and molecular speed in gas behavior. It explores the Maxwell-Boltzmann distribution, highlighting the significance of understanding molecular properties and behavior for effective gas utilization. The script also touches on the concept of molecular velocity and its impact on gas distribution, emphasizing the need for a deep understanding of these principles for practical applications.
Takeaways
- 🔬 The script discusses the molecular speed and distribution in gases, emphasizing the importance of understanding the behavior of molecules at different temperatures and states.
- 📈 It highlights the significance of Maxwell's distribution law, which describes the distribution of molecular speeds in a gas.
- 🌡️ The temperature's effect on gas molecules is a key point, with higher temperatures leading to a broader range of molecular speeds.
- 🧪 The script touches on the concept of molecular velocity and how it can be calculated using statistical mechanics.
- 📚 There is mention of an educational aspect, possibly a lecture or a series, that covers the topic of gas molecules and their behavior.
- 📉 The loss of distribution and the concept of 'Maxwellian distribution loss' are discussed, suggesting a decrease in the uniformity of molecular speed distribution.
- 📝 The importance of considering the molecular concentration and how it relates to the volume and density of the gas is emphasized.
- 🔍 The script delves into the measurement of molecular velocity and the challenges associated with accurately determining the properties of gas molecules.
- 📉 The concept of 'distribution loss' is revisited, indicating that the script may be discussing a phenomenon or process that leads to an uneven distribution of molecular speeds.
- 🔢 The script mentions equations and mathematical functions that are crucial for understanding the behavior of gas molecules, suggesting a technical or scientific approach to the topic.
- 🌐 It concludes with a call to action for subscribing to a channel or lecture series, indicating that the content is part of an educational series on the physics of gases.
Q & A
What is the main topic discussed in the script?
-The main topic discussed in the script appears to be related to the molecular properties of gases, their distribution, and the factors affecting their behavior in various conditions.
What is the significance of the number of molecules in the context of the script?
-The number of molecules is significant as it relates to the concentration, distribution, and the behavior of gases, which are central to understanding the script's scientific discussion.
What is the role of temperature in the script's discussion about gases?
-Temperature plays a crucial role as it affects the velocity and distribution of gas molecules. It is mentioned in the context of thermal equilibrium and the behavior of gases when they are heated or cooled.
What does the script suggest about the relationship between molecular speed and temperature?
-The script suggests that there is a direct relationship between molecular speed and temperature, where an increase in temperature leads to an increase in the speed of gas molecules.
What is the concept of 'molecular velocity' as discussed in the script?
-Molecular velocity refers to the speed at which gas molecules move. The script discusses it in the context of how it varies from zero to infinity and how it is influenced by temperature.
How does the script relate the distribution of molecular speed to the behavior of gases?
-The script relates the distribution of molecular speed to the behavior of gases by explaining that the range of speeds (from zero to a large number) affects how gases behave under different conditions.
What is the importance of understanding the 'molecular concentration' as mentioned in the script?
-Understanding molecular concentration is important because it helps in determining the density and behavior of gases, which is essential for various scientific and industrial applications.
What factors are mentioned in the script that could affect the distribution of gas molecules?
-Factors mentioned in the script that could affect the distribution of gas molecules include temperature, pressure, and the presence of other substances or compounds.
How does the script discuss the concept of 'perfect gas'?
-The script discusses the concept of 'perfect gas' in the context of an idealized state where gas molecules have no volume and move with no intermolecular forces, which is important for theoretical calculations and understanding gas behavior.
What is the relevance of 'statistical mechanics' in the script's discussion?
-Statistical mechanics is relevant in the script's discussion as it provides a framework for understanding the distribution and behavior of gas molecules based on statistical properties rather than individual interactions.
What does the script imply about the importance of education in understanding gas properties?
-The script implies that education is crucial for understanding the complex properties and behaviors of gases, suggesting that knowledge in this area is fundamental and should be further explored.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade Now5.0 / 5 (0 votes)