A Level Chemistry Revision "Relative Atomic Mass"
Summary
TLDRThis video teaches the concepts of relative isotopic mass and relative atomic mass, explaining how they are calculated using isotope data. It highlights that relative isotopic mass is a whole number without units, and relative atomic mass is a weighted mean mass also without units, derived from the abundance of isotopes.
Takeaways
- 🔬 Atoms have extremely small masses, making their direct measurement challenging.
- 📌 Scientists use relative mass to compare atomic masses, with carbon-12 as the reference point.
- 🌐 Carbon-12 is assigned a mass of exactly 12, and other atoms' masses are measured relative to 1/12 of its mass.
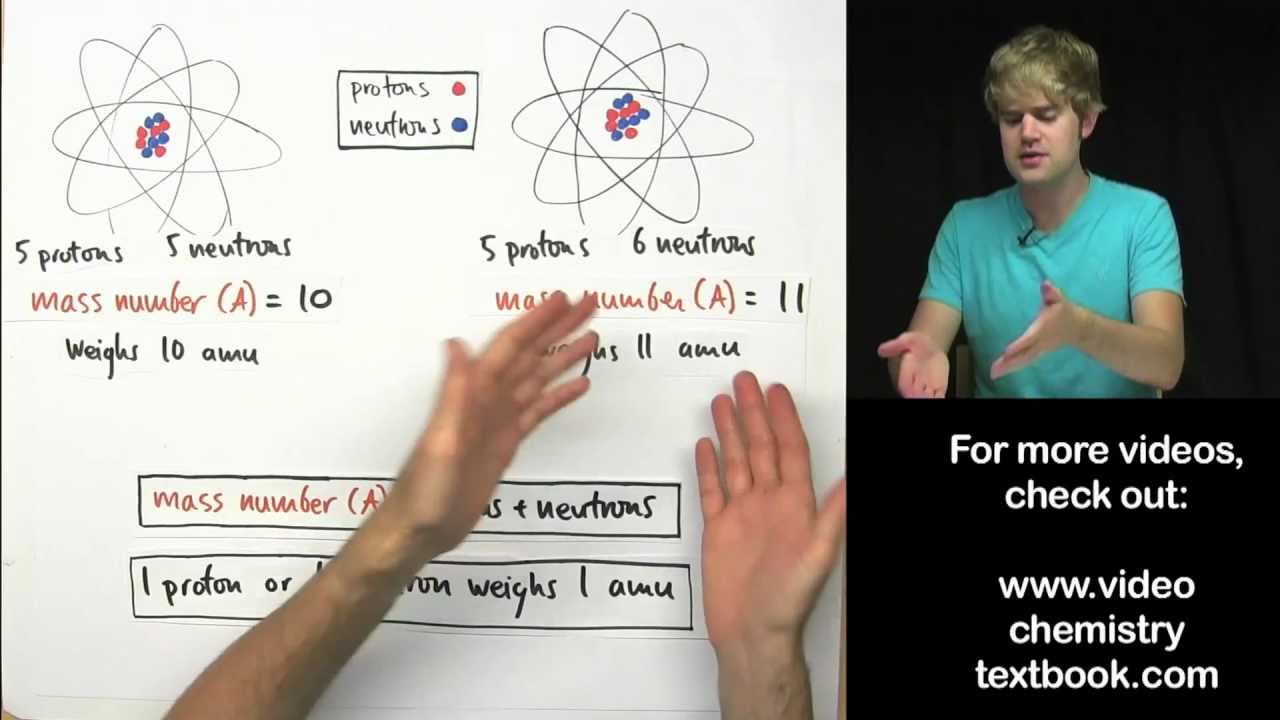
- 🧪 Isotopes are atoms of the same element with different numbers of neutrons, resulting in different masses.
- 🏗️ Relative isotopic mass is the mass of an isotope compared to 1/12 the mass of carbon-12.
- 📊 Relative isotopic mass is always a whole number and has no units.
- 📚 The periodic table lists the relative atomic mass of elements, which is calculated from the relative isotopic masses.
- 📈 Relative atomic mass is the weighted mean mass of an element's isotopes, taking into account their abundance.
- 🧩 The relative atomic mass is not always a whole number, reflecting the average mass based on isotopic abundance.
- 📘 To calculate relative atomic mass, use the equation involving the relative isotopic masses and the percent abundance of each isotope.
Q & A
What is the main topic of the video?
-The main topic of the video is the concept of relative isotopic mass and relative atomic mass, and how to calculate relative atomic mass from isotope data.
Why are relative masses used in chemistry?
-Relative masses are used in chemistry because atoms are extremely small and their actual masses are very tiny, making it impractical to use absolute masses in calculations.
What is the reference isotope for relative mass in chemistry?
-The reference isotope for relative mass in chemistry is carbon-12.
How is the mass of carbon-12 defined in terms of relative mass?
-In chemistry, the mass of carbon-12 is defined as exactly 12, and 1/12 of its mass is counted as 1 relative mass unit.
What are isotopes and how do they differ?
-Isotopes are atoms of the same element with a different number of neutrons, which results in different masses for each isotope.
What is the definition of relative isotopic mass?
-Relative isotopic mass is defined as the mass of an atom of an isotope compared with 1/12 the mass of carbon-12.
Why is the relative isotopic mass always a whole number?
-The relative isotopic mass is always a whole number because it is a ratio comparing the mass of an isotope to a fraction of the mass of carbon-12, which is a fixed reference.
What is the formula used to calculate the relative atomic mass of an element?
-The formula to calculate the relative atomic mass is the sum of the products of the relative isotopic masses and their respective percent abundances, divided by 100.
Why are relative atomic masses not whole numbers on the periodic table?
-Relative atomic masses are not whole numbers on the periodic table because they are weighted means based on the abundance of each isotope, resulting in a non-integer value.
Can you provide an example of calculating the relative atomic mass using the given formula?
-Yes, for chlorine with isotopes chlorine-35 (75% abundance) and chlorine-37 (25% abundance), the relative atomic mass is calculated as (35 * 0.75) + (37 * 0.25) = 35.5.
What is the significance of the relative atomic mass in the periodic table?
-The relative atomic mass in the periodic table represents the average atomic mass of an element, taking into account the natural abundance of its isotopes, and is used in chemical calculations.
Outlines

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифMindmap

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифKeywords

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифHighlights

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифTranscripts

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифПосмотреть больше похожих видео

Relative Atomic Mass | Properties of Matter | Chemistry | FuseSchool

MASSA ATOM RELATIF : KIMIA SMA KELAS 10

Kimia SMA - Stokiometri (1) - Massa Atom Relatif, Massa Molekul Relatif, Ar, Mr (D)

Basic Definitions | Atoms, Molecules and Stoichiometry | 9701 AS Chemistry Urdu/Hindi
A Level Chemistry Revision "Relative Molecular Mass and Relative Formula Mass"
What's the Difference between Mass Number and Atomic Mass?
5.0 / 5 (0 votes)
