How to Find Oxidation Numbers (Rules and Examples)
Summary
TLDRThis video introduces the fundamental rules for determining oxidation numbers in chemistry. It covers key concepts like the rule for neutral compounds where oxidation numbers sum to zero, the distinctions between oxidation numbers and ionic charges, and specific element behaviors (e.g., fluorine always being -1, hydrogen’s oxidation number depending on bonding). The video also explains how to approach complex cases such as sulfur in sulfate ions and chlorine in chlorate ions, with practical examples throughout. By the end, viewers are encouraged to practice further to solidify their understanding of oxidation numbers.
Takeaways
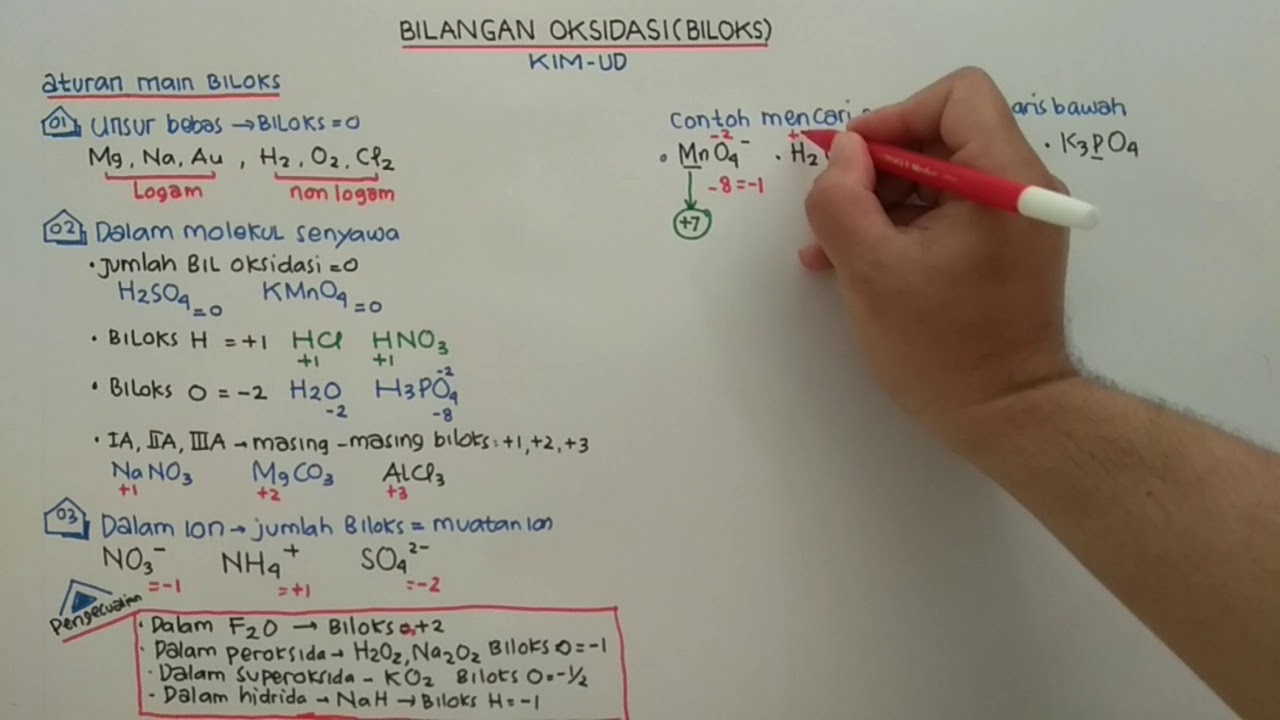
- 😀 Oxidation numbers in a neutral compound must add up to zero.
- 😀 In ions, the oxidation numbers of atoms must add up to the charge of the ion.
- 😀 The oxidation number of carbon in CO2 is +4, as determined by balancing the oxidation numbers of oxygen (which is -2).
- 😀 Oxidation numbers and ionic charges are not the same; for example, nitrogen's ionic charge is always -3, but its oxidation number can vary.
- 😀 The oxidation number for free elements (monatomic substances) is always zero.
- 😀 Fluorine always has an oxidation number of -1 in compounds.
- 😀 Group 1 elements (alkali metals) have an oxidation number of +1, Group 2 elements (alkaline earth metals) have +2, and aluminum has +3.
- 😀 Hydrogen typically has an oxidation number of +1 when bonded to nonmetals and -1 when bonded to metals.
- 😀 Oxygen generally has an oxidation number of -2, except when bonded to fluorine or in peroxides where it can be -1.
- 😀 When rules don't apply, use a specific order of rules to determine oxidation numbers.
Q & A
What is the rule for assigning oxidation numbers in neutral compounds?
-In neutral compounds, the sum of the oxidation numbers of all atoms must equal zero.
How do you determine the oxidation number of carbon in CO₂?
-In CO₂, oxygen has an oxidation number of -2. Since there are two oxygen atoms, their total oxidation number is -4. To balance this to zero, the oxidation number of carbon must be +4.
What happens when you calculate the oxidation number in ions?
-For ions, the sum of the oxidation numbers must equal the charge of the ion. For example, in SO₄²⁻ (sulfate ion), the oxidation number of sulfur is +6 to balance the charge of -2 from four oxygen atoms.
What is the difference between oxidation numbers and ionic charges?
-Oxidation numbers and ionic charges are not the same. The ionic charge is the overall charge of the ion, while oxidation numbers are assigned to individual atoms within a compound based on specific rules.
What is the oxidation number of fluorine in compounds?
-Fluorine always has an oxidation number of -1 in compounds.
What is the oxidation number of hydrogen when bonded to nonmetals?
-When hydrogen is bonded to nonmetals, its oxidation number is +1.
How does the oxidation number of oxygen change in peroxides?
-In peroxides, the oxidation number of oxygen is -1, which is an exception to the usual oxidation number of oxygen, which is typically -2.
What are the oxidation numbers for elements in Groups 1, 2, and 13 of the periodic table?
-Elements in Group 1 have an oxidation number of +1, elements in Group 2 have an oxidation number of +2, and aluminum (Al) in Group 13 has an oxidation number of +3.
What is the oxidation number of oxygen in the compound F₂O?
-In F₂O, oxygen has an oxidation number of +2, since fluorine always has an oxidation number of -1, and the compound is neutral.
What should you do if none of the standard rules for assigning oxidation numbers apply?
-If none of the standard rules apply, you can use more specific guidelines in a particular order to determine the oxidation numbers of the elements in the compound.
Outlines

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифMindmap

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифKeywords

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифHighlights

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифTranscripts

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифПосмотреть больше похожих видео
5.0 / 5 (0 votes)