OSMOSE E OS URSINHOS DE GELATINA - STEAM PROJECT QUÍMICA E BIOLOGIA
Summary
TLDRIn this engaging video, Alessandra Rocha explains the fascinating science behind gummy bears' behavior in water, focusing on the concept of osmosis. By conducting an experiment with two cups—one with just water and the other with a saltwater solution—she shows how the gummy bears expand in plain water and shrink in the saltwater. The video explores how water moves in and out of the gummy bear's gelatin shell due to concentration differences, helping to explain osmosis in a fun, easy-to-understand way. Viewers are encouraged to explore osmosis further, with relatable examples like the effect of salt on salad vegetables.
Takeaways
- 😀 Gummy bears grow in water and shrink in saltwater due to osmosis, a process where water moves in and out of substances to balance concentrations.
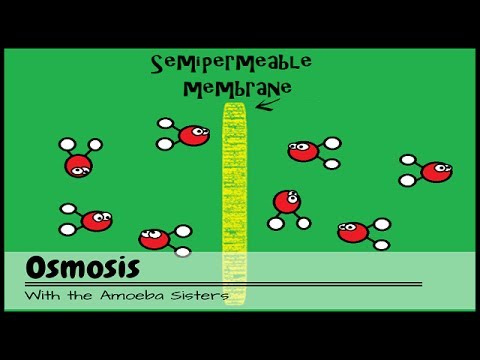
- 😀 Osmosis occurs when water passes through a semi-permeable membrane, like the gelatin in gummy bears, to equalize concentrations of solute.
- 😀 In pure water, the gummy bear swells as water enters to dilute the sugar inside, causing it to increase in size.
- 😀 In saltwater, water leaves the gummy bear to balance the high concentration of salt outside, causing it to shrink.
- 😀 The semi-permeable membrane of the gelatin allows water to enter and exit but blocks other substances, which is why osmosis occurs.
- 😀 The movement of water during osmosis does not require energy, making it a passive process.
- 😀 Gummy bears are made of a concentrated sugar solution inside their gelatin shell, which is why osmosis affects them in different solutions.
- 😀 Osmosis is a key process in living cells, helping to maintain balance by regulating the movement of water in and out of cells.
- 😀 The same process of osmosis can be observed in vegetables, such as tomatoes and lettuce, when salt and vinegar are added.
- 😀 The video encourages viewers to explore the concept of osmosis by observing how their salad changes after adding salt and vinegar.
Q & A
What happens to gummy bears when they are placed in water?
-When gummy bears are placed in water, they absorb the water and swell up, increasing in size due to the process of osmosis.
What is osmosis?
-Osmosis is the process where water moves through a semi-permeable membrane, from an area of low solute concentration to an area of high solute concentration, in an attempt to balance the concentrations.
Why does a gummy bear swell in water?
-A gummy bear swells in water because the water moves into the gummy bear, attempting to dilute the sugar solution inside, increasing the gummy bear's volume.
What is the difference between the two cups in the experiment?
-The first cup contains only water, while the second cup contains a solution of water and salt. The gummy bear behaves differently in each cup due to the difference in solute concentration.
What happens to the gummy bear in the saline solution?
-In the saline solution, the gummy bear shrinks because water moves out of the gummy bear to balance the higher concentration of salt outside.
Why do gummy bears behave differently in water and saline solutions?
-Gummy bears behave differently because the concentration of solutes in the surrounding liquid affects how water moves in and out of the gummy bear through osmosis. In water, it swells, and in saline solution, it shrinks.
What role does the semi-permeable membrane of the gummy bear play in osmosis?
-The semi-permeable membrane of the gummy bear allows water to move in and out freely, but it restricts the movement of larger solutes like sugar and salt, facilitating the osmotic process.
How does osmosis help in maintaining balance in cells?
-Osmosis helps cells maintain balance by regulating the movement of water to ensure that the concentration of solutes inside the cell is in equilibrium with the outside environment.
What real-life example of osmosis is mentioned in the script?
-A real-life example of osmosis mentioned in the script is the effect of salt and vinegar on salad vegetables like tomatoes and leafy greens, which absorb or lose water, affecting their texture and appearance.
How does this experiment help us understand the process of osmosis in biology?
-This experiment helps us understand osmosis in biology by demonstrating how water moves in and out of a semi-permeable membrane, which is similar to what happens in cells and plants to maintain equilibrium.
Outlines

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифMindmap

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифKeywords

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифHighlights

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифTranscripts

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифПосмотреть больше похожих видео

How Are Rain Droplets Formed? | WATER CYCLE | The Dr Binocs Show | Peekaboo Kidz

How Is Gelatin Made? | How Pig Skins Become Your Favorite Gummy Candies

How to Draw Phase Diagrams and What they Mean! | Doc Physics

Potato experiment | Osmosis | Biology

Diversity of Bodies & Sizes (but mostly crabs): Crash Course Zoology #3
(OLD VIDEO) Osmosis
5.0 / 5 (0 votes)
