MASSA ATOM RELATIF : KIMIA SMA KELAS 10
Summary
TLDRThis video explains the concept of atomic mass and its measurement using atomic mass units. It discusses how the mass of atoms is determined through comparison with the carbon-12 isotope and introduces the concept of relative atomic mass. The video also covers calculations using mass spectrometry to determine the relative atomic mass of elements with multiple isotopes. Examples include calculations involving carbon-12, magnesium, boron, and gallium, illustrating how atomic mass is used to quantify elements and their isotopes, along with the concept of isotope abundance in nature.
Takeaways
- 😀 The size of an atom is extremely small, with even a single grain of sand containing around 10^16 atoms.
- 😀 Atoms cannot be weighed directly using conventional scales due to their minuscule size.
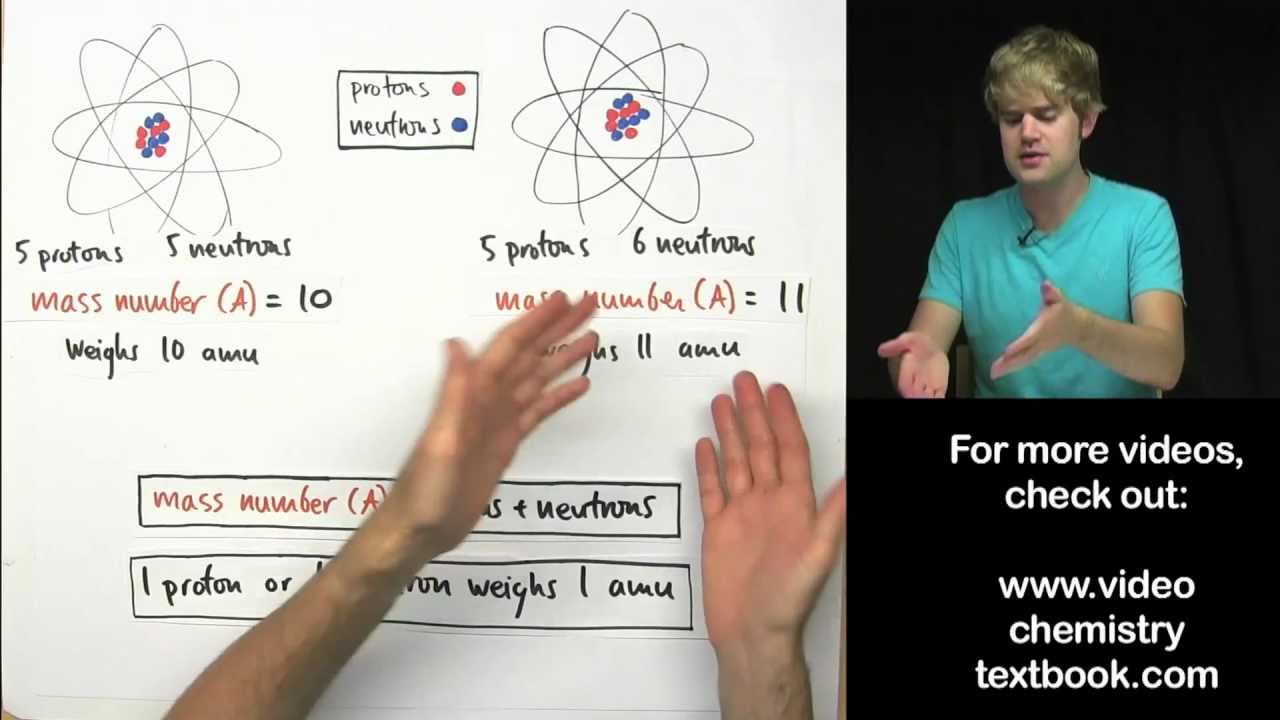
- 😀 The mass of an atom is typically determined relative to another atom, specifically using the carbon-12 atom as a standard.
- 😀 In 1961, IUPAC established the carbon-12 atom as the standard for atomic mass relative to other atoms.
- 😀 Atomic mass is defined as the ratio of the mass of an atom to the mass of one atom of carbon-12.
- 😀 The atomic mass unit (AMU) is defined as 1/12th of the mass of a carbon-12 atom, which equals approximately 1.66 × 10^-24 grams.
- 😀 The relative atomic mass (AR) of an element is calculated by multiplying the mass of each isotope by its natural abundance and summing the results.
- 😀 Mass spectrometers are used to accurately determine the atomic mass and isotopic distribution of elements.
- 😀 If the mass of one atom of element X is 2.66 × 10^-23 grams, its relative atomic mass (AR) can be calculated as 16.4, compared to carbon-12.
- 😀 The mass of 500 atoms of magnesium can be calculated using its atomic mass, which is 4 × 10^-23 grams per atom, resulting in a total mass of 2 × 10^-20 grams.
- 😀 For elements with multiple isotopes, the relative atomic mass is determined by considering both the mass and the relative abundance of each isotope.
- 😀 Boron has two isotopes, boron-10 and boron-11, with respective masses of 10.013 AMU and 11.011 AMU, and relative abundances of 19.10% and 80.90%. The resulting relative atomic mass of boron is 10.81.
Q & A
What is the definition of atomic mass?
-Atomic mass is the mass of a single atom of an element, measured in atomic mass units (amu). It is typically determined by comparing the atom's mass to that of a standard reference, such as the carbon-12 isotope.
How many atoms are contained in a single grain of sand?
-A single grain of sand, which is visible to the naked eye, can contain about 10^16 atoms due to the small size of atoms.
What is the standard atom used for determining relative atomic mass?
-The standard atom used for determining relative atomic mass is the carbon-12 (C12) isotope, which has been set by IUPAC.
How is relative atomic mass (Ar) mathematically defined?
-Relative atomic mass (Ar) is defined as the ratio of the average mass of an atom of an element to the mass of a carbon-12 atom. Mathematically, Ar = (mass of atom) / (1/12 mass of C12 atom).
How is atomic mass unit (AMU) defined?
-An atomic mass unit (AMU) is defined as 1/12th the mass of a carbon-12 atom, which equals approximately 1.66 × 10^-24 grams.
How can the mass of atoms other than carbon-12 be measured?
-The mass of atoms other than carbon-12 can be measured experimentally using a mass spectrometer, which provides accurate data about the isotopes of an element and their natural abundances.
What is the formula used to calculate relative atomic mass when an element has multiple isotopes?
-The formula for relative atomic mass when an element has multiple isotopes is: Ar = (M1 × Z1) + (M2 × Z2) + ... + (Mn × Zn), where M is the isotope mass and Z is its natural abundance.
What is the relative atomic mass of element X if its atom mass is 2.66 × 10^-23 grams?
-The relative atomic mass of element X can be calculated by dividing its atomic mass by 1/12th of the mass of a carbon-12 atom. For element X, the relative atomic mass is 16.4.
How can the mass of 500 atoms of magnesium be calculated?
-To calculate the mass of 500 atoms of magnesium, first determine the mass of one magnesium atom (4 × 10^-23 grams) and multiply by 500, resulting in a total mass of 2 × 10^-20 grams.
How is the relative atomic mass of boron determined from its isotopes?
-The relative atomic mass of boron is calculated using the formula for atomic mass: Ar = (M1 × Z1) + (M2 × Z2). For boron, using the given isotopes boron-10 and boron-11, the calculated relative atomic mass is approximately 10.8.
How is the abundance of isotopes in gallium determined given its relative atomic mass?
-The abundance of gallium isotopes is determined using the equation for relative atomic mass. For gallium, with isotopes Ga-69 and Ga-71, it is found that the abundance of Ga-69 is 60% and Ga-71 is 40%.
Outlines

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифMindmap

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифKeywords

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифHighlights

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифTranscripts

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тариф5.0 / 5 (0 votes)