Part 1 Kesetimbangan Kimia: Reaksi Irreversible, Reaksi Reversible dan Konsep Kesetimbangan Dinamis.
Summary
TLDRThis video provides a comprehensive introduction to chemical equilibrium, explaining the difference between irreversible (one-way) and reversible (two-way) reactions. It covers the concept of dynamic equilibrium, where reactions continue at equal rates, leading to constant concentrations of reactants and products. The video also distinguishes between homogeneous and heterogeneous equilibrium, depending on whether the substances involved are in the same or different phases. Additionally, the equilibrium constants Kc and Kp are introduced to describe the balance between reactants and products. Overall, the video aims to simplify complex equilibrium concepts, making them accessible and engaging for learners.
Takeaways
- 😀 **Irreversible Reactions (One-way)**: Reactants convert into products that cannot be reversed, such as NaOH + HCl → NaCl + H₂O.
- 😀 **Reversible Reactions (Two-way)**: Products can reform reactants, represented by a double arrow (⇌), e.g., 2SO₂ + O₂ ⇌ 2SO₃.
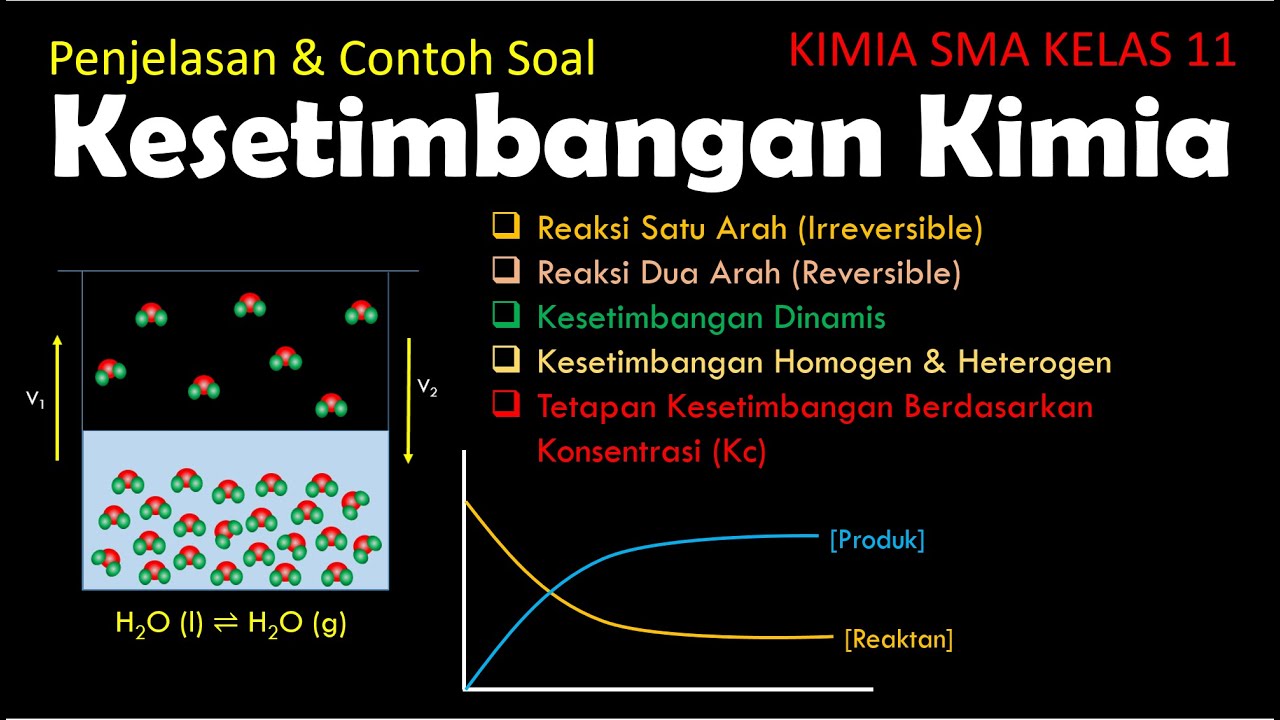
- 😀 **Dynamic Equilibrium**: A state where the rate of the forward and reverse reactions are equal, and the concentrations of reactants and products remain constant.
- 😀 **Evaporation and Condensation Example**: In a closed system, the rates of evaporation and condensation become equal, leading to dynamic equilibrium.
- 😀 **Key Characteristic of Dynamic Equilibrium**: There is no observable change in the system's macroscopic properties, though microscopic changes continue.
- 😀 **Closed System Requirement**: For dynamic equilibrium to be achieved, the system must be closed with constant temperature and pressure.
- 😀 **Homogeneous Equilibrium**: All reactants and products are in the same physical state (e.g., gas or liquid), like N₂ + 3H₂ ⇌ 2NH₃.
- 😀 **Heterogeneous Equilibrium**: Reactants and products are in different states (solid, liquid, gas), such as C + H₂ ⇌ CO + H₂.
- 😀 **Concentration Changes at Equilibrium**: Three possible scenarios can occur: products > reactants, products < reactants, or products = reactants.
- 😀 **Equilibrium Constants (Kc and Kp)**: Kc represents the equilibrium constant based on concentration, while Kp is based on partial pressures of gases.
- 😀 **Dynamic Equilibrium Is Continuous**: Reactions continue to occur at the microscopic level, even when no visible changes are observed in the macroscopic properties of the system.
Q & A
What is the difference between irreversible (one-way) and reversible (two-way) reactions?
-Irreversible reactions produce products that cannot revert back into reactants, represented with a single arrow (→), while reversible reactions allow products to convert back into reactants, represented with a double arrow (⇌).
Can you give an example of an irreversible reaction from the script?
-An example of an irreversible reaction is the reaction between NaOH (aqueous) and HCl (aqueous), which produces NaCl (aqueous) and H2O (liquid).
What is the definition of dynamic equilibrium?
-Dynamic equilibrium occurs when two opposing reactions (e.g., evaporation and condensation) proceed at the same rate, meaning the concentrations of reactants and products remain constant over time, despite the reactions continuing.
What are the key characteristics of dynamic equilibrium?
-The key characteristics include: 1) the reaction is reversible and continuous, 2) it occurs in a closed system with constant temperature and pressure, 3) the rate of the forward reaction equals the rate of the reverse reaction, and 4) macroscopic changes do not occur, though microscopic changes are happening at the particle level.
What is meant by a system being 'closed' in dynamic equilibrium?
-A closed system means that no matter (reactants or products) can enter or leave, so the reaction reaches equilibrium within the confines of the system, allowing the concentration of reactants and products to stabilize.
What happens to the concentrations of reactants and products during dynamic equilibrium?
-During dynamic equilibrium, the concentrations of reactants and products remain constant over time, as the rates of the forward and reverse reactions are equal.
What is the difference between homogeneous and heterogeneous equilibrium?
-In homogeneous equilibrium, all substances involved are in the same phase (e.g., all gases or all liquids), whereas in heterogeneous equilibrium, the substances are in different phases (e.g., a solid, liquid, and gas).
Provide an example of a homogeneous equilibrium from the script.
-An example of a homogeneous equilibrium is the reaction between N2 (gas) and H2 (gas) to form NH3 (gas), where all reactants and products are in the gas phase.
Can you give an example of a heterogeneous equilibrium from the script?
-An example of a heterogeneous equilibrium is the reaction between solid C (carbon) and gas H2 to produce CO (gas) and H2 (gas).
What are the possible outcomes regarding the concentration of reactants and products at equilibrium?
-At equilibrium, the concentration of products may be greater than reactants, less than reactants, or equal to reactants. However, the concentrations will remain constant over time once equilibrium is reached.
Outlines

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифMindmap

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифKeywords

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифHighlights

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифTranscripts

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифПосмотреть больше похожих видео
KESETIMBANGAN KIMIA ( KIMIA SMA KELAS 11 )

11 клас. Хімія. Необоротні та оборотні хімічні реакції. Хімічна рівновага. Принцип Ле Шательє

Kesetimbangan Kimia • Part 1: Konsep, Hukum, Tetapan Kesetimbangan Kc dan Kp

KESETIMBANGAN KIMIA KELAS 11_PART 1

6. Chemical Reactions (Part 3) (3/5) (Cambridge IGCSE Chemistry 0620 for 2023, 2024 & 2025)

IGCSE CHEMISTRY REVISION [Syllabus 7]- Chemical Reactions
5.0 / 5 (0 votes)
