Metallic Bonding and its properties 1
Summary
TLDRMetal atoms, with few valence electrons, form a crystalline lattice where these electrons are delocalized, creating a 'sea' that allows for electrical conductivity. This metallic bonding, driven by electrostatic forces between the delocalized electrons and cations, gives metals their characteristic strength and high melting points, distinguishing them from ionic solids.
Takeaways
- 🔬 Metal atoms have few electrons in their valence shell, which are weakly held.
- 🔗 Metal atoms are grouped together in a crystalline lattice structure.
- 🌐 The properties of metals differ significantly from those of ionic solids.
- ⚡ Solid metals conduct electricity due to the delocalized valence electrons.
- 🌊 These electrons form a 'sea' that can move freely through the lattice.
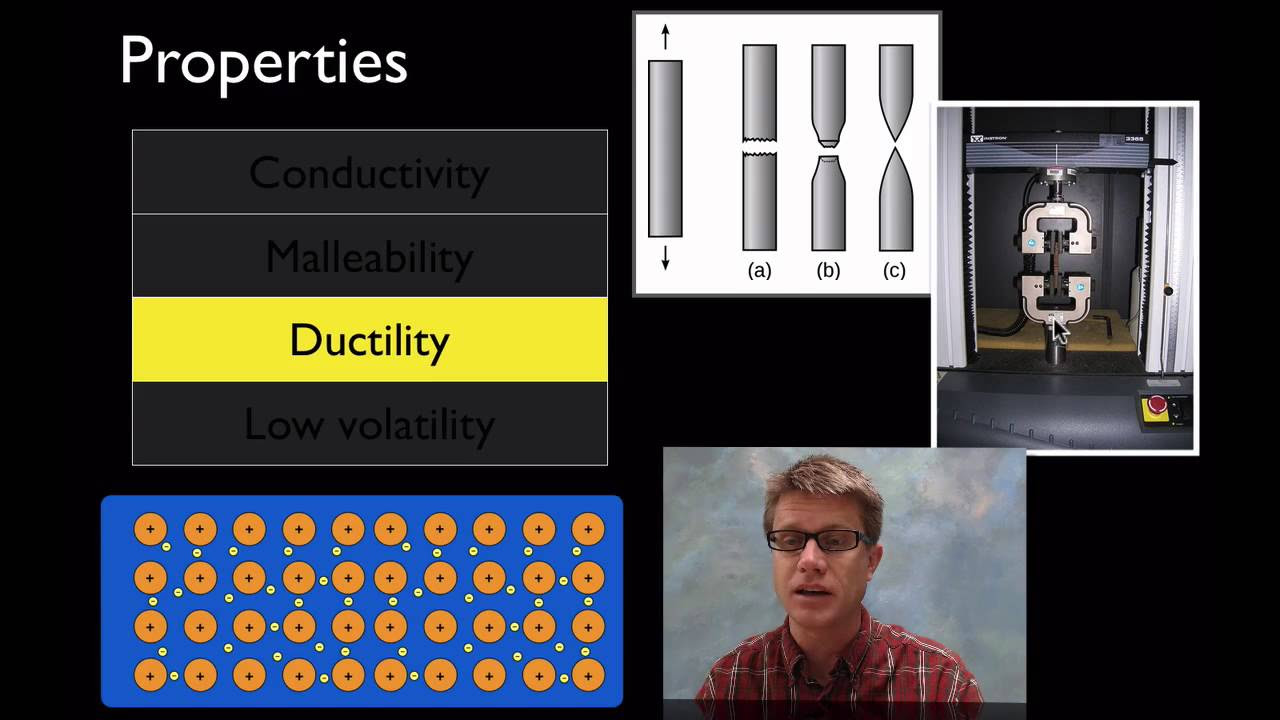
- 🔝 The lattice is held together by strong electrostatic forces between the delocalized electrons and metal cations.
- 🔗 This type of bonding is known as metallic bonding, which acts in all directions.
- 🔥 The high melting points of most metals are indicative of the strength of metallic bonds.
- 🔁 The constant movement of electrons in metallic bonding allows for conductivity and strength.
- 🔍 The metallic bond's ability to overcome repulsion between cations highlights its power.
Q & A
Why do metal atoms have few electrons in their valence shell?
-Metal atoms typically have few electrons in their valence shell because they are positioned towards the left and center of the periodic table, which is where elements with fewer valence electrons are found.
Why are the valence electrons of metal atoms weakly held?
-The valence electrons of metal atoms are weakly held due to the larger atomic size and the distance of these electrons from the nucleus, which results in a weaker attractive force between the electrons and protons.
What is a crystalline lattice structure?
-A crystalline lattice structure is a highly ordered, repeating three-dimensional arrangement of atoms, ions, or molecules that forms the basis of a solid's structure.
How do the properties of metals differ from ionic solids?
-Metals differ from ionic solids in that they are malleable and ductile, have high electrical and thermal conductivity, and are typically shiny, whereas ionic solids are brittle, poor conductors of electricity and heat, and often lack luster.
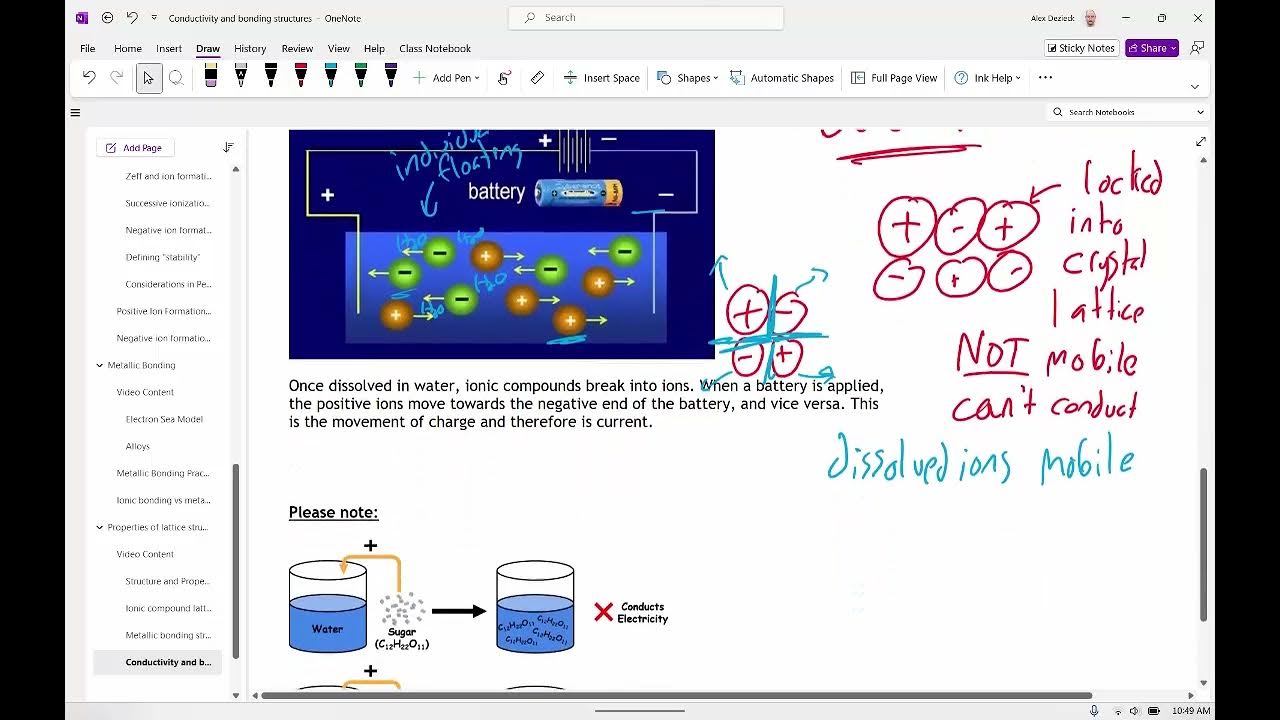
Why can solid metals conduct electricity?
-Solid metals conduct electricity because the valence electrons are delocalized and can move freely through the metal lattice, allowing for the flow of electric current.
What is meant by delocalized electrons in the context of metals?
-Delocalized electrons in metals refer to the valence electrons that are not tightly bound to any single atom but are free to move throughout the entire lattice, contributing to the metal's conductivity.
What is the term for the cloud of delocalized valence electrons surrounding the metal cations?
-The term for the cloud of delocalized valence electrons surrounding the metal cations is the 'sea of electrons' or 'electron cloud'.
How does metallic bonding differ from other types of bonding?
-Metallic bonding differs from other types of bonding in that it involves a delocalized sea of electrons that are shared among all the metal atoms, rather than discrete pairs of electrons being shared between two atoms as in covalent bonding, or the electrostatic attraction between ions as in ionic bonding.
Why are the melting points of most metals high?
-The melting points of most metals are high because the metallic bond, which involves the strong electrostatic attraction between the delocalized electrons and the positively charged metal cations, requires significant energy to overcome.
What is the role of the electrostatic force in metallic bonding?
-The electrostatic force in metallic bonding plays a crucial role by holding the positively charged metal cations together through the attraction to the negatively charged sea of delocalized electrons.
How does the movement of electrons contribute to the properties of metals?
-The constant movement of electrons in metals contributes to their properties by allowing for the transfer of energy and charge, which results in metals' ability to conduct electricity and heat, and their malleability and ductility.
Outlines

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифMindmap

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифKeywords

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифHighlights

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифTranscripts

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тариф5.0 / 5 (0 votes)