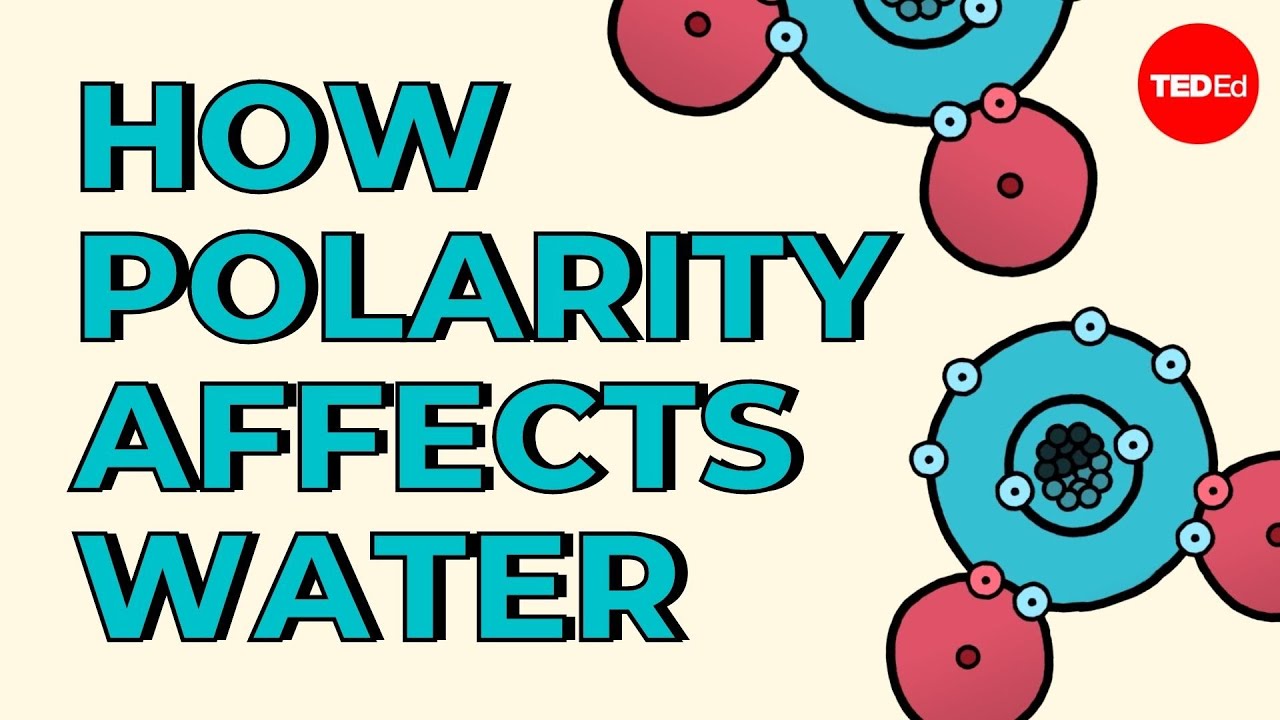
Das Kugelwolkenmodell und die Valenzelektronen
Summary
TLDRThis script delves into the molecular structure of water, explaining why H2O consists of two hydrogen atoms bonded to one oxygen atom. It introduces the concept of valence electrons and the periodic table, illustrating how elements' outer electron configurations determine their bonding behavior. The video simplifies complex chemistry by discussing electron shells and the spatial arrangement of atoms in molecules, like methane and water, using the Lewis dot structure. It concludes by addressing the dipole nature of water and its anomalous properties, making chemistry accessible and intriguing.
Takeaways
- 💧 Water is a fundamental element known as H2O, consisting of two hydrogen atoms and one oxygen atom.
- 🔬 The structure of water involves two hydrogen atoms due to chemical bonding principles.
- ⚛️ Valence electrons are key to understanding chemical bonds; they are the electrons involved in bonding.
- 🧪 The number of valence electrons for an element can be determined by its position in the periodic table.
- 📚 Elements in the same column (group) of the periodic table have the same number of valence electrons.
- 🧫 Oxygen, in the sixth group, has six valence electrons, but not all form bonds.
- 🌐 The electron cloud model helps explain molecular structure and atomic bonding.
- 🌀 Electron clouds or orbitals can hold up to two electrons and form bonds by overlapping with others.
- 🧩 Molecules are structured to keep electron clouds as far apart as possible due to repulsion.
- 🔗 In water (H2O), oxygen forms bonds with two hydrogen atoms, resulting in its bent molecular shape.
Q & A
What is the chemical formula for water?
-The chemical formula for water is H2O, which consists of two hydrogen atoms and one oxygen atom.
Why does water have the formula H2O instead of H or another number of hydrogen atoms?
-Water has the formula H2O because of the valency and electron configuration of oxygen, which can form two bonds with hydrogen atoms, resulting in a stable molecule.
What is the concept of valency in chemistry?
-Valency, also known as valence, refers to the number of chemical bonds an atom can form with other atoms. It is determined by the number of valence electrons an atom has.
How can the periodic table help us determine the valency of elements?
-The periodic table organizes elements into groups, with elements in the same group having the same number of valence electrons, which corresponds to their valency.
What is the significance of the electron shell model in understanding atomic structure and chemical bonding?
-The electron shell model helps us understand the arrangement of electrons around an atomic nucleus and how these electron shells participate in chemical bonding to form molecules.
How many electrons can fit in the first electron shell of an atom?
-The first electron shell can accommodate up to two electrons, forming a single electron cloud or orbital.
What is the difference between a single and a double bond in terms of electron cloud overlap?
-A single bond involves the overlap of one electron cloud from each of the two bonding atoms, while a double bond involves the overlap of two electron clouds from each atom.
Why do electrons in an atom prefer to be as far apart as possible?
-Electrons prefer to be as far apart as possible to minimize electron-electron repulsion, which is a result of their negative charges repelling each other.
How does the electron cloud model explain the spatial structure of molecules?
-The electron cloud model explains the spatial structure of molecules by showing how the electron clouds arrange themselves to maximize distance from each other, which in turn dictates the molecule's geometry.
What is the molecular geometry of methane (CH4) according to the electron cloud model?
-According to the electron cloud model, the molecular geometry of methane (CH4) is tetrahedral, with the four hydrogen atoms' electron clouds positioned as far apart as possible around the central carbon atom.
Why is water considered a polar molecule?
-Water is considered a polar molecule because of the difference in electronegativity between oxygen and hydrogen, which results in an uneven distribution of electron density and creates a molecular dipole.
Outlines

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードMindmap

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードKeywords

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードHighlights

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードTranscripts

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレード5.0 / 5 (0 votes)