The Quantum Theory of the Atom | Chapter 7 - General, Organic, and Biological Chemistry
Summary
TLDRThis video delves into the revolutionary shift in physics from classical theories to quantum mechanics, explaining how light and matter behave as both waves and particles. Starting with historical experiments like blackbody radiation and the photoelectric effect, the script explores pivotal discoveries by Planck, Einstein, and Bohr. It then tackles the complex ideas of wave-particle duality, Heisenberg’s uncertainty principle, and Schrödinger’s equation, revealing how these concepts define atomic structure. With analogies and real-world applications, the script brings clarity to quantum mechanics, ultimately highlighting its essential role in modern chemistry and technology.
Takeaways
- 😀 Light behaves as both a wave and a particle, a concept known as wave-particle duality, which revolutionized our understanding of physics.
- 😀 Max Planck introduced the idea of quantized energy, proposing that energy is not continuous, but instead comes in discrete packets called quanta.
- 😀 The photoelectric effect, explained by Einstein, provided strong evidence for light's particle nature. The energy of a photon depends on its frequency, not brightness.
- 😀 Niels Bohr's model of the atom proposed quantized energy levels for electrons, explaining atomic spectra but was limited to hydrogen atoms.
- 😀 Bohr’s atomic model could not explain the behavior of atoms with more than one electron due to the complex interactions between them.
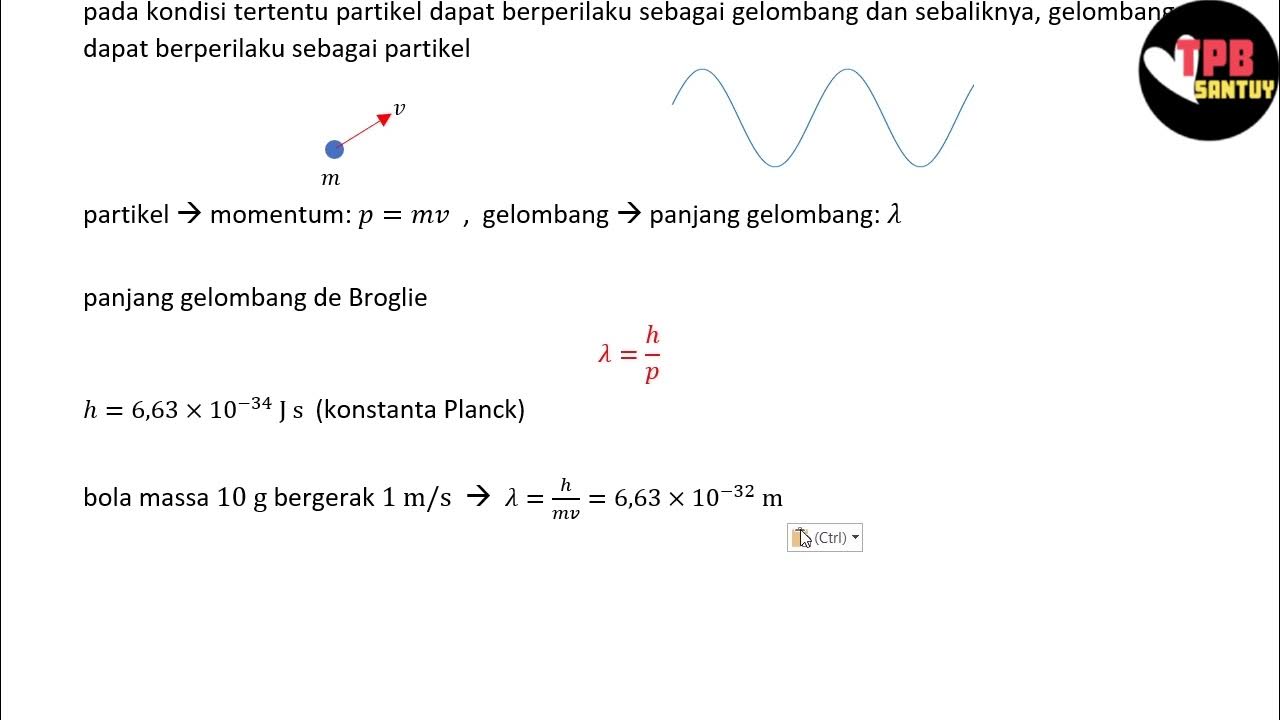
- 😀 Louis de Broglie proposed that matter, like electrons, can also behave as waves, introducing the concept of matter waves.
- 😀 The wave-like behavior of electrons was confirmed experimentally through electron diffraction, which demonstrated that electrons could diffract like light waves.
- 😀 Heisenberg's uncertainty principle states that we cannot simultaneously know both the position and momentum of a particle with absolute precision.
- 😀 The modern quantum mechanical model replaces Bohr's orbits with orbitals, which are probabilistic descriptions of where an electron is likely to be found.
- 😀 Schrödinger’s wave equation describes the electron as a matter wave and provides the framework for understanding atomic orbitals, defined by three quantum numbers (N, L, mL).
Q & A
What is the main difference between classical models of the atom and the quantum mechanical model?
-Classical models, like Bohr's atom, treated electrons as particles moving in fixed orbits around the nucleus, with energy levels quantized. The quantum mechanical model, however, treats electrons as existing in probabilistic 'clouds' rather than fixed paths, and their positions and momenta can only be described in terms of probabilities.
How does the uncertainty principle influence our understanding of electrons in atoms?
-Heisenberg's uncertainty principle states that it's impossible to know both the exact position and momentum of a particle, like an electron, simultaneously. This means we can never precisely predict an electron's trajectory, only the probability of finding it in a certain region of space.
What does the term 'probability density' mean in the context of the quantum mechanical model?
-Probability density refers to the square of the wave function, which gives the likelihood of finding an electron at a particular point in space. It’s not the exact location, but a measure of where the electron is most likely to be found.
Why are radial probability distribution plots important in quantum mechanics?
-Radial probability distribution plots show the total probability of finding an electron at a certain distance from the nucleus. They provide insight into where an electron is most likely to be found, considering the entire volume of space around the nucleus, and they often peak at a certain distance due to the geometry of the atom.
What role do quantum numbers play in identifying electron states?
-Quantum numbers are used to describe the energy, shape, and orientation of an electron's orbital. They provide a unique 'address' for each electron, specifying its energy level, orbital shape, and orientation in three-dimensional space.
What is the principal quantum number (n), and how does it relate to an electron's energy and size?
-The principal quantum number (n) is a positive integer that indicates the energy level or shell of an electron. A higher value of n corresponds to a larger orbital, generally farther from the nucleus, and a higher energy state.
How does the angular momentum quantum number (l) affect the shape of an orbital?
-The angular momentum quantum number (l) determines the shape of the orbital. The value of l can range from 0 to n-1, and each value of l corresponds to a different orbital shape, such as spherical (s orbitals), dumbbell-shaped (p orbitals), and more complex shapes for d and f orbitals.
What is the difference between an orbital and a subshell in quantum mechanics?
-An orbital is a specific region within an atom where an electron is likely to be found, defined by a set of quantum numbers. A subshell refers to a group of orbitals that share the same value of the angular momentum quantum number (l), such as the s, p, d, or f subshells.
Why do different sublevels (s, p, d, f) have different numbers of orbitals?
-The number of orbitals in a subshell is determined by the value of the magnetic quantum number (ml), which ranges from -l to +l, including zero. This gives 2l+1 orbitals in each subshell. For example, the s subshell has 1 orbital, the p subshell has 3 orbitals, the d subshell has 5 orbitals, and the f subshell has 7 orbitals.
How does the energy of orbitals in multi-electron atoms differ from that in a hydrogen atom?
-In a hydrogen atom, all orbitals with the same principal quantum number (n) have the same energy, a concept known as degenerate orbitals. In multi-electron atoms, electron-electron repulsions and shielding effects cause orbitals within the same shell (same n) to have different energies, with s orbitals being the lowest in energy, followed by p, d, and f orbitals.
Outlines

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードMindmap

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードKeywords

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードHighlights

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードTranscripts

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレード関連動画をさらに表示

Davisson-Germer Experiment & Wave-Particle Duality

Física Quântica (Dr. Quantum) Fenda Dupla - Dublado PT

Por Que Precisamos da Dualidade Onda-Partícula?

Quantum 101 Episode 1: Wave Particle Duality Explained
Dualisme Gelombang-Partikel | Fenomena Kuantum | Part 1 | Fisika Dasar

Konsep Dasar Fisika Modern-Kuantum
5.0 / 5 (0 votes)
