Radioatividade: Alfa, Beta e Gama.
Summary
TLDRThis educational video explores the nature of radiation, focusing on alpha, beta, and gamma particles. The instructor explains how each type of radiation helps unstable nuclei achieve stability: alpha particles reduce mass, beta particles adjust neutron-to-proton ratios, and gamma rays release excess energy. The lesson covers symbols, mass and atomic number changes, and real-world examples like uranium-235 decay and carbon-14 dating. Viewers also learn about the penetrating abilities of each radiation type, with gamma rays being the most dangerous. Practical tips for calculating decay sequences are provided, making complex nuclear concepts accessible and engaging for learners.
Takeaways
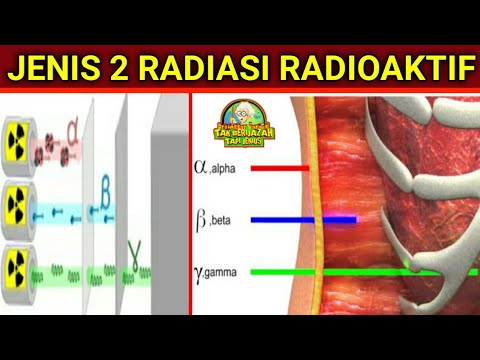
- 😀 The video discusses the three main types of radiation: alpha (α), beta (β), and gamma (γ) radiation, along with their characteristics and effects.
- 😀 Alpha particles are heavy, positively charged, and characteristic of very heavy nuclei; they reduce the mass of the nucleus by 4 and the atomic number by 2.
- 😀 Beta particles are electrons (β⁻) or positrons (β⁺) emitted by nuclei with an excess of neutrons or protons, changing the atomic number by ±1 but not the mass.
- 😀 Gamma rays are high-energy electromagnetic radiation, with no mass or charge, and result from nuclei losing excess energy.
- 😀 Radiation occurs to achieve nuclear stability, reducing excess mass, excess neutrons, or excess energy in the nucleus.
- 😀 Alpha particles are similar to helium nuclei, beta particles are similar to electrons, and gamma rays are purely energy.
- 😀 Penetrating power varies: alpha particles are least penetrating, beta particles are moderately penetrating, and gamma rays are highly penetrating, requiring thick shielding.
- 😀 Carbon-14 decays via beta emission, converting into nitrogen to reduce neutron excess, illustrating real-life radioactive decay applications.
- 😀 For radioactive decay calculations, first balance alpha emissions (top numbers) and then beta emissions (bottom numbers) to satisfy mass and atomic number conservation.
- 😀 The principle 'nothing is lost, nothing is created, everything is transformed' is central to understanding radiation and nuclear decay.
- 😀 Understanding radiation types is important for both theoretical knowledge and practical applications, including safety measures and nuclear chemistry problems.
Q & A
What are the three main types of radiation discussed in the video?
-The three main types of radiation are alpha (α) particles, beta (β) particles, and gamma (γ) rays.
What is the main purpose of a nucleus emitting radiation?
-The purpose is to achieve stability by reducing excess mass, neutrons, or energy in the nucleus.
How does alpha decay affect a nucleus's mass and atomic number?
-Each alpha particle emitted reduces the mass of the nucleus by 4 units and the atomic number by 2 units.
What type of nuclei typically emit alpha particles?
-Very heavy nuclei typically emit alpha particles as part of their process to reach stability.
How does beta decay change a nucleus?
-Beta decay changes the atomic number by ±1 while keeping the mass approximately the same, helping to correct a neutron-proton imbalance.
What is the difference between a beta particle and a positron?
-A beta particle (β−) has a negative charge, similar to an electron, while a positron (β+) has a positive charge. Both are forms of beta decay.
Why are gamma rays considered highly penetrating?
-Gamma rays are high-frequency electromagnetic radiation with no mass or charge, allowing them to penetrate matter easily; they require thick lead or concrete for shielding.
In a decay sequence, what is the recommended order for calculating emissions?
-First, calculate the number of alpha particles to adjust the mass, then use beta particles to adjust the atomic number.
How does carbon-14 decay into nitrogen-14?
-Carbon-14 emits a beta particle to reduce excess neutrons, converting its atomic number from 6 to 7, transforming it into nitrogen-14 while keeping the mass constant.
What analogy is used to describe alpha particles?
-Alpha particles are similar to helium nuclei, containing 2 protons and 2 neutrons, essentially making them a helium nucleus.
How does radiation penetration relate to safety considerations?
-Radiation with higher penetrating power, like gamma rays, is more dangerous and requires stronger shielding, whereas alpha particles are the least penetrating and can be stopped by paper or skin.
What is meant by 'nothing is lost, everything is transformed' in nuclear decay?
-This principle means that during radiation emission, the total mass-energy is conserved; particles are transformed into other particles or energy, but not destroyed.
Outlines

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードMindmap

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードKeywords

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードHighlights

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードTranscripts

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレード5.0 / 5 (0 votes)