Trends in Ionisation Energies | AS-Level Chemistry
Summary
TLDRThis video explains the concept of ionization energy, which is the energy required to remove electrons from gaseous atoms to form ions. It covers first, second, and successive ionization energies, highlighting how factors like atomic radius, shielding, and nuclear charge influence them. The tutorial explores periodic trends, showing that ionization energy decreases down groups due to increasing distance and shielding, and generally increases across periods due to stronger nuclear charge. It also explains exceptions, such as drops between Be-B and N-O, caused by orbital type and electron pairing. Clear examples and diagrams make understanding these trends accessible and exam-ready.
Takeaways
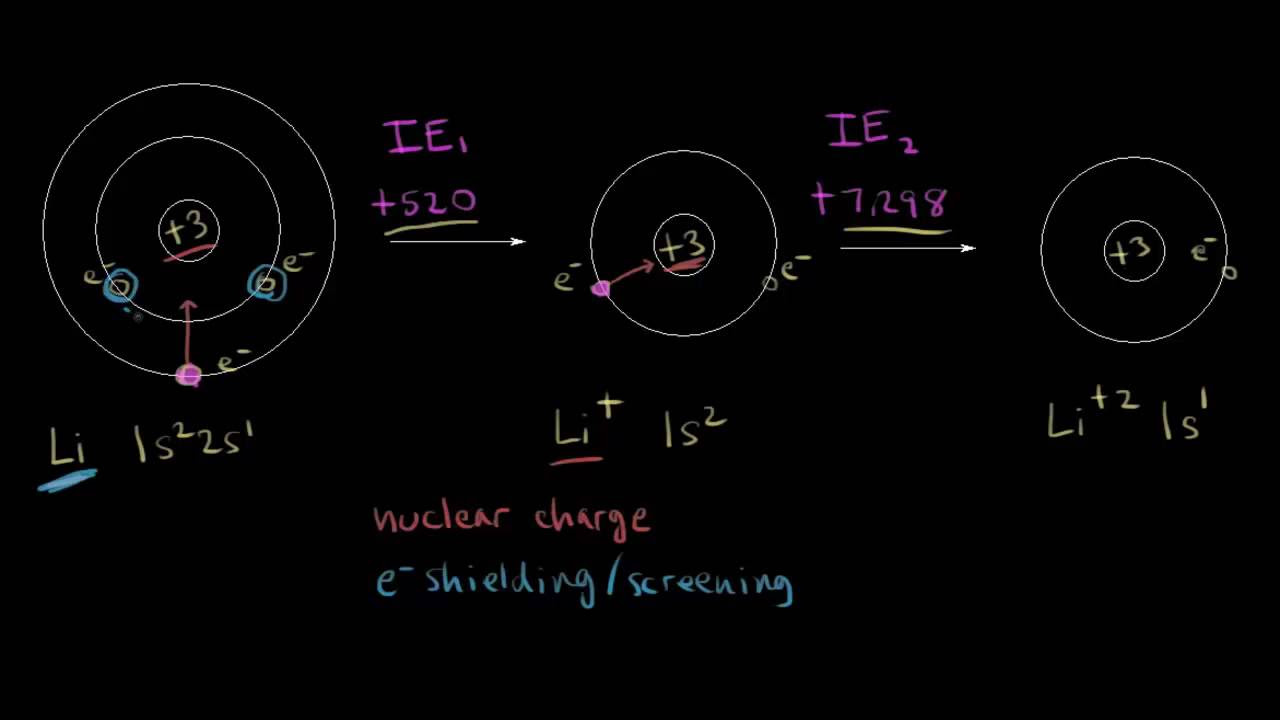
- 😀 Ionization energy (IE) is the energy required to remove one mole of electrons from one mole of gaseous atoms, forming one mole of cations.
- 😀 The first ionization energy refers to removing the first electron from an atom, forming a 1+ ion, while subsequent ionization energies remove additional electrons from increasingly charged ions.
- 😀 Factors affecting ionization energy include atomic radius (distance), shielding by inner electrons, and nuclear charge (number of protons).
- 😀 Increasing distance or shielding lowers ionization energy, whereas increasing nuclear charge raises ionization energy.
- 😀 Down a group in the periodic table, ionization energy decreases because increased distance and shielding outweigh the increase in nuclear charge.
- 😀 Across a period, ionization energy generally increases due to higher nuclear charge and smaller atomic radius, while shielding remains mostly constant.
- 😀 Exceptions to the general trend across a period occur due to subshell configurations: Be → B drops because B’s outermost electron is in a higher-energy p-orbital, and N → O drops due to paired electron repulsion in oxygen’s p-orbital.
- 😀 Successive ionization energies increase progressively, with large jumps indicating the removal of core electrons.
- 😀 The trends in ionization energy help predict element behavior, including valence electron counts and periodic table placement.
- 😀 Understanding electron configurations and orbital arrangements is essential for explaining variations and exceptions in ionization energy trends.
Q & A
What is the definition of the first ionization energy?
-The first ionization energy is the energy required to remove one mole of electrons from one mole of gaseous atoms to form one mole of gaseous +1 ions.
How does the second ionization energy differ from the first?
-The second ionization energy involves removing an electron from a +1 ion to form a +2 ion, so it generally requires more energy than the first because the electron is being removed from an already positively charged ion.
What are the three main factors that affect ionization energy?
-The three main factors are: 1) Distance (atomic radius) – the further the outer electron from the nucleus, the easier it is to remove. 2) Shielding – inner electrons repel outer electrons, making them easier to remove. 3) Nuclear charge – more protons increase attraction, making electrons harder to remove.
How does ionization energy change as you move down a group in the periodic table?
-Ionization energy decreases as you move down a group because the distance from the nucleus and shielding increase, making it easier to remove the outermost electron. Although nuclear charge increases, the effects of distance and shielding dominate.
Why does ionization energy generally increase across a period?
-Across a period, the number of protons (nuclear charge) increases while shielding remains approximately constant. This pulls electrons closer to the nucleus, reducing atomic radius and making it harder to remove electrons, so ionization energy increases.
Why are there small drops in ionization energy between certain elements across a period?
-Drops occur due to subshell configurations. For example, the drop from beryllium to boron happens because boron's outer electron is in a higher-energy p subshell with more shielding. The drop from nitrogen to oxygen occurs because oxygen has paired electrons in a p orbital, and paired electrons repel each other, making one easier to remove.
What role does electron shielding play in ionization energy?
-Electron shielding reduces the effective attraction between the nucleus and outermost electron. More inner electron shells create more shielding, making it easier to remove the outer electron and thus lowering ionization energy.
How does nuclear charge influence ionization energy?
-A higher nuclear charge (more protons in the nucleus) increases the attraction between the nucleus and electrons, pulling them closer. This makes it more difficult to remove an electron, increasing ionization energy.
How can successive ionization energies help us understand an atom's electron configuration?
-By removing electrons one by one and measuring the energy required, successive ionization energies reveal when electrons are removed from inner, more tightly bound shells. Large jumps indicate the removal of core electrons, providing insight into the number of valence electrons.
Why does the ionization energy of oxygen decrease compared to nitrogen despite following the general trend across a period?
-Oxygen has one paired electron in its p orbital, which experiences repulsion from the other electron in the same orbital. This repulsion makes it easier to remove that electron, causing a lower ionization energy than nitrogen, where all outer electrons are unpaired.
What mnemonic can help remember the effects of distance, shielding, and nuclear charge on ionization energy?
-You can use the mnemonic 'DSC' – Distance, Shielding, Charge. Increased Distance and Shielding lower ionization energy, whereas increased Charge raises it.
Outlines

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードMindmap

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードKeywords

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードHighlights

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードTranscripts

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレード関連動画をさらに表示

A Level Chemistry Revision "First Ionisation Energy"

AQA A-Level Chemistry - Ionisation Energies
First and second ionization energy | Atomic structure and properties | AP Chemistry | Khan Academy

5.3 Electron Configuration and Periodic Properties (1/2)
Coulomb's Law

GCSE Chemistry - Formation of Ions #13
5.0 / 5 (0 votes)
