Proses Sulfonasi Senyawa Aromatik
Summary
TLDRIn this video, Imelda, an expert in chemistry, explains the sulfonation process in aromatic compounds, focusing on the reaction of sulfuric acid with aromatic molecules. She describes how sulfuric acid reacts to form electrophilic species like SO3, which then interact with aromatic compounds, leading to the substitution of hydrogen with sulfonic acid groups. Imelda also touches on the role of activating and deactivating groups in influencing the reactivity of the aromatic compounds, using specific examples and emphasizing electron resonance effects during the reaction. The explanation concludes with the final product and its significance.
Takeaways
- 😀 The sulfonation reaction in aromatic compounds involves the use of sulfuric acid as a key reactant.
- 😀 Sulfuric acid (H2SO4) has a polar bond due to its electronegativity, which creates a positive and negative charge in the molecule.
- 😀 The reaction begins with the formation of the electrophile SO3+ through the interaction of sulfuric acid's components.
- 😀 The electrophilic sulfur trioxide (SO3) reacts with aromatic compounds, specifically with benzene or its derivatives.
- 😀 The reaction is facilitated by the presence of an activating group (e.g., OH) that donates electrons and a deactivating group (e.g., NO2) that withdraws electrons.
- 😀 The activating group (OH) increases electron density on the aromatic ring, making it more reactive.
- 😀 The deactivating group (NO2) withdraws electron density, making the aromatic ring less reactive to electrophilic substitution.
- 😀 The sulfonation process involves breaking and forming bonds on the aromatic ring, resulting in substitution at specific carbon positions.
- 😀 During the reaction, electrons flow between the aromatic ring and the electrophile, causing resonance effects that stabilize the intermediate carbocation.
- 😀 The final product of the sulfonation reaction is the aromatic compound bonded with a sulfonic acid group (-SO3H), as seen in the case of amoxicillin.
Q & A
What is the main focus of the sulfonation reaction in aromatic compounds?
-The main focus of the sulfonation reaction in aromatic compounds is the substitution of a hydrogen atom in the aromatic ring with a sulfonic acid group (–SO₃H), facilitated by sulfuric acid.
How does sulfuric acid act in the sulfonation reaction?
-Sulfuric acid acts as an electrophile in the sulfonation reaction. It undergoes protonation to form the electrophilic species SO₃⁺, which attacks the electron-rich aromatic ring.
What role does the sulfuric acid molecule play in the reaction mechanism?
-Sulfuric acid provides the electrophilic species SO₃⁺, which reacts with the aromatic compound. The protonated sulfuric acid facilitates the generation of this electrophile, which then undergoes an electrophilic aromatic substitution.
Why is SO₃⁺ formed in the reaction, and how is it generated?
-SO₃⁺ is formed due to the protonation of sulfuric acid (H₂SO₄). This makes the sulfur atom more electrophilic, which leads to the formation of SO₃⁺, a highly reactive species capable of attacking the aromatic compound.
What is the role of electron-rich and electron-withdrawing groups in the sulfonation reaction?
-Electron-rich groups, like OH, activate the aromatic ring towards electrophilic substitution, making it more reactive. In contrast, electron-withdrawing groups, like nitro groups, deactivate the ring, making it less reactive to the electrophile SO₃⁺.
How does the aromatic ring react with the electrophile SO₃⁺ in the sulfonation process?
-The aromatic ring, which contains electron-rich π bonds, reacts with the electrophile SO₃⁺. This results in the substitution of one of the hydrogen atoms on the ring with a sulfonic acid group (–SO₃H).
What is the significance of resonance in the sulfonation reaction?
-Resonance helps stabilize the carbocation intermediate formed during the reaction. The delocalization of electrons through resonance makes the reaction more favorable and facilitates the formation of the final product.
What is the final product of the sulfonation reaction described in the script?
-The final product of the sulfonation reaction is an aromatic compound with a sulfonic acid group (–SO₃H) substituted onto the benzene ring.
What happens to the electron distribution in the aromatic ring during the reaction?
-During the reaction, the electron density of the aromatic ring is redistributed. The activating groups increase electron density, making the ring more reactive, while the deactivating groups withdraw electron density, making the ring less reactive.
Why does the sulfonation reaction occur with the involvement of SO₃⁺ and sulfuric acid?
-The reaction occurs because SO₃⁺ is a strong electrophile that reacts with the electron-rich aromatic ring, leading to a substitution of a hydrogen atom with a sulfonic acid group. Sulfuric acid plays a crucial role by generating SO₃⁺ and assisting in the electrophilic substitution process.
Outlines

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードMindmap

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードKeywords

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードHighlights

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードTranscripts

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレード関連動画をさらに表示

Substitusi Elektrofilik Aromatik: Sulfonasi
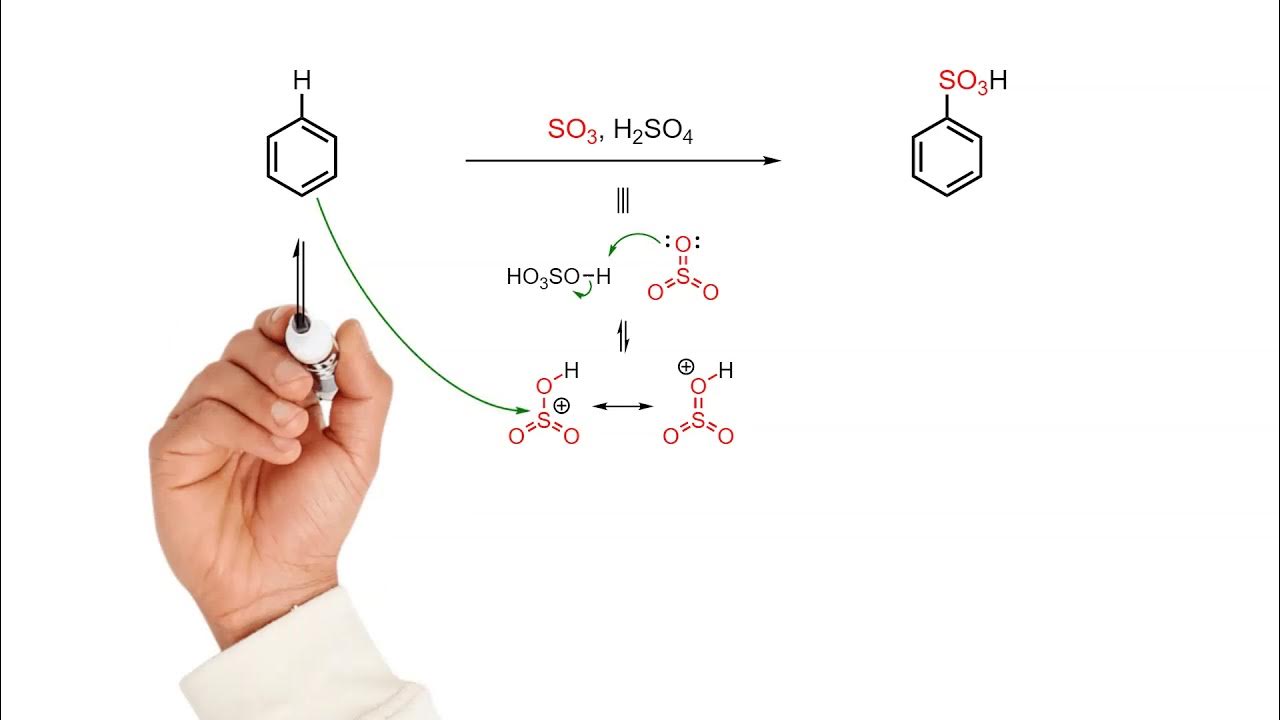
Sulfonation of benzene

Benzene Reaction | Nitration Reaction: Reaction Mechanism, Nitration Method, Nitration Application

Naming benzene derivatives introduction | Aromatic Compounds | Organic chemistry | Khan Academy

Práctica de laboratorio-Hidrocarburos

Reaksi Benzena | Reaksi Alkilasi Friedel-Crafts: Mekanisme Reaksi dan Batasan Alkilasi [LENGKAP]
5.0 / 5 (0 votes)
