PERUBAHAN ENTALPI STANDAR ( BAB TERMOKIMA - MAPEL KIMIA KELAS 11 )
Summary
TLDRThis video explains the four types of standard enthalpy changes: formation, decomposition, combustion, and neutralization. It details the conditions under which these enthalpies are measured, focusing on the reactions' coefficients and their corresponding heat values. Key examples include the formation of water, decomposition of acetylene, combustion of acetylene, and neutralization reactions with NaOH and H2SO4. The video emphasizes the importance of adjusting coefficients to match standard conditions, ensuring the correct representation of enthalpy changes for one mole of substance. These concepts are fundamental in thermodynamics, particularly in calculating and understanding heat changes in chemical reactions.
Takeaways
- 😀 The standard enthalpy change is divided into four types: formation, decomposition, combustion, and neutralization.
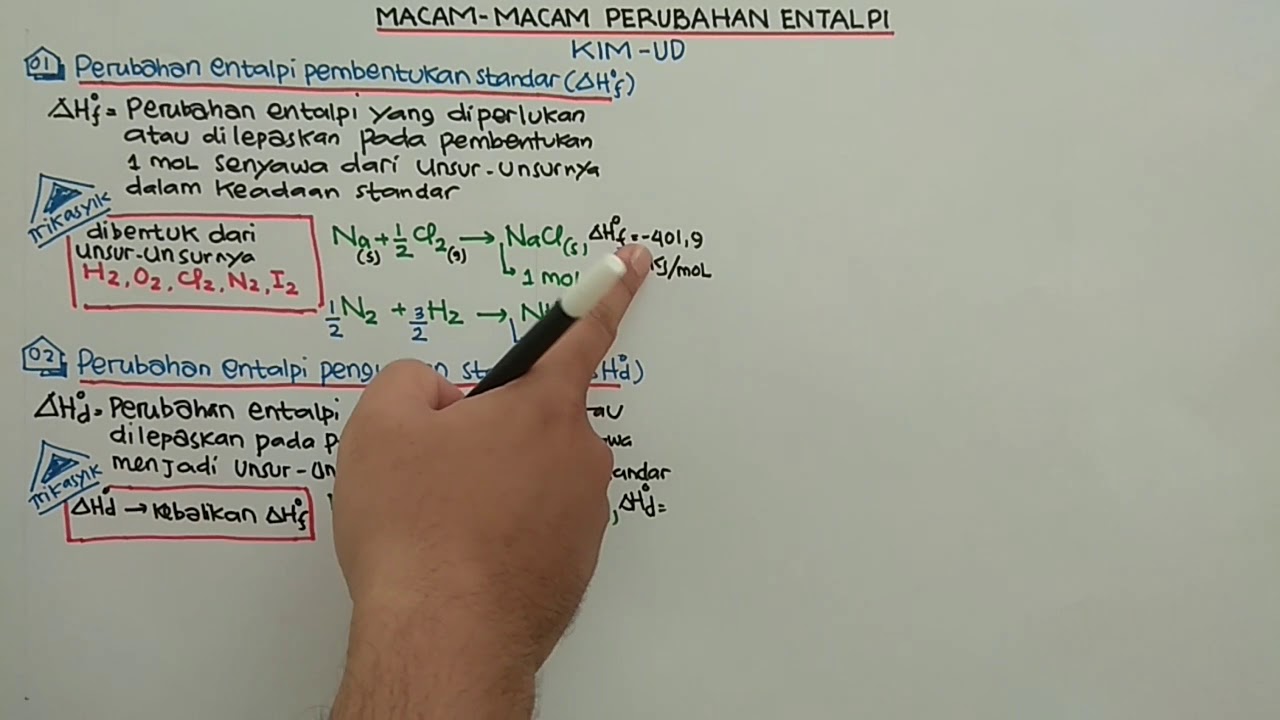
- 😀 Standard enthalpy of formation (ΔHf°) is the enthalpy change when one mole of a substance is formed from its elements under standard conditions (25°C and 1 atm).
- 😀 A reaction is considered a standard formation reaction if the product's coefficient is one and the reactants are the elements in their natural states.
- 😀 The standard enthalpy of formation of H2O (liquid) is -285.85 kJ/mol, indicating it’s formed from H2 and O2 in their elemental forms.
- 😀 A reaction with a coefficient of 2, like 2 C2H2 forming 4 carbon and 2 hydrogen gas, is not a standard formation reaction unless coefficients are adjusted.
- 😀 The standard enthalpy of decomposition (ΔHd°) refers to the enthalpy change when one mole of a compound breaks down into its elemental forms, and it is the reverse of the enthalpy of formation.
- 😀 Decomposition reactions follow the rule that the reactants must be elements, and if the coefficient is not 1, adjustments need to be made (e.g., dividing the reaction by 2).
- 😀 Standard enthalpy of combustion (ΔHc°) refers to the enthalpy change when one mole of a substance undergoes complete combustion with oxygen, and the coefficient of the reactant must be 1.
- 😀 For the combustion of C2H2 (acetylene), the standard enthalpy of combustion is -1256 kJ/mol, meaning the equation must have a coefficient of 1 for C2H2.
- 😀 Standard enthalpy of neutralization (ΔHn) refers to the heat released when 1 mole of an acid and 1 mole of a base neutralize each other under standard conditions.
- 😀 In the neutralization of NaOH with H2SO4, the enthalpy change is -200 kJ for 2 moles of NaOH, but to standardize it to 1 mole, the value is divided by 2, resulting in -100 kJ/mol.
Q & A
What is the definition of standard enthalpy of formation (ΔHf°)?
-The standard enthalpy of formation (ΔHf°) refers to the change in enthalpy when one mole of a substance is formed from its elements in their standard states at 25°C and 1 atmosphere of pressure.
Why is the coefficient of the product in a standard enthalpy of formation reaction always 1?
-In a standard enthalpy of formation reaction, the coefficient of the product must be 1 because it represents the formation of one mole of the substance from its elements, ensuring the reaction reflects the correct enthalpy change for one mole of the product.
Can you provide an example of a reaction that demonstrates standard enthalpy of formation?
-An example is the reaction: H₂ (gas) + ½ O₂ (gas) → H₂O (liquid), with ΔHf° = -285.85 kJ/mol. This is the formation of one mole of liquid water from hydrogen and oxygen.
What happens when a reaction has a coefficient greater than 1 for the product in the formation reaction?
-If the coefficient of the product is greater than 1, the reaction does not represent a standard enthalpy of formation. To adjust, the reaction should be divided by the coefficient to ensure the correct molar formation.
What is the relationship between standard enthalpy of formation (ΔHf°) and standard enthalpy of decomposition (ΔHd°)?
-The standard enthalpy of decomposition (ΔHd°) is the reverse of the standard enthalpy of formation (ΔHf°). The enthalpy change for the decomposition of a substance is equal in magnitude but opposite in sign to its formation enthalpy.
What does the standard enthalpy of combustion (ΔHc°) represent?
-The standard enthalpy of combustion (ΔHc°) is the enthalpy change when one mole of a substance undergoes complete combustion with oxygen under standard conditions (25°C and 1 atm).
Can you provide an example of a reaction showing standard enthalpy of combustion?
-An example of a standard combustion reaction is: C₂H₂ (gas) + 5/2 O₂ (gas) → 2 CO₂ (gas) + H₂O (gas), with ΔHc° = -1256 kJ/mol. This represents the complete combustion of one mole of acetylene gas.
What is the importance of having a coefficient of 1 for the reactant in combustion reactions?
-The coefficient of 1 for the reactant in combustion reactions ensures that the reaction represents the combustion of one mole of the substance. If the coefficient is greater, the enthalpy value needs to be adjusted accordingly.
How is the standard enthalpy of neutralization (ΔHn) defined?
-The standard enthalpy of neutralization (ΔHn) is the enthalpy change when one mole of an acid is neutralized by one mole of a base, or vice versa, under standard conditions.
Why must the coefficients in neutralization reactions be adjusted to one mole for the standard enthalpy of neutralization?
-The coefficients in neutralization reactions must be adjusted to one mole for standard enthalpy of neutralization because the definition refers to the enthalpy change for the neutralization of one mole of acid or base. If the coefficient is greater, the enthalpy value must be divided accordingly.
Outlines

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードMindmap

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードKeywords

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードHighlights

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードTranscripts

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレード5.0 / 5 (0 votes)