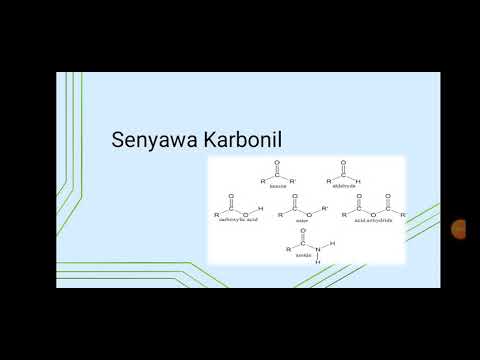
Carbonyl acidity at the alpha position
Summary
TLDRThis video explains the concept of acidity and pKa, focusing on the alpha position of various carbonyl compounds. It discusses how the presence of multiple carbonyl groups (dicarbonyls) increases acidity due to greater resonance stabilization of the resulting anion. The video compares mono- and dicarbonyl compounds, emphasizing that dicarbonyl compounds are more acidic and reactive. It also highlights how resonance and electron donation play a role in determining acidity, with examples of different carbonyl derivatives like esters, ketones, and amides. The ranking from most acidic to least is discussed, with dicarbonyl compounds being the most reactive.
Takeaways
- 😀 The lower the pKa, the more acidic a compound is, indicating stronger acidity.
- 😀 The alpha position of carbonyl compounds (next to the carbonyl group) is key to their acidity due to resonance stabilization of the resulting anion.
- 😀 Dicarbonyl compounds are more acidic than monocarbonyl compounds because their conjugate bases are more stabilized by resonance between the two carbonyl groups.
- 😀 Mono carbonyl compounds include esters, ketones, and amides, each with different resonance stabilization abilities, affecting their acidity.
- 😀 Amides are the least acidic among monocarbonyl compounds because nitrogen donates electrons, reducing resonance stabilization.
- 😀 Esters are more acidic than ketones due to better resonance stabilization of the conjugate base.
- 😀 Ketones are more acidic than amides but less acidic than esters due to less effective resonance stabilization.
- 😀 Dicarbonyl compounds, such as diketones, are highly reactive and acidic due to strong resonance stabilization between the two carbonyl groups.
- 😀 A ketone and ester combination (C) is more acidic than a diester (F), but less so than a diketone (E).
- 😀 For dicarbonyl compounds, the order of acidity from most to least acidic is: diketone > ketone + ester > diester.
Q & A
What is the relationship between pKa and acidity in carbonyl compounds?
-The lower the pKa value, the more acidic the compound is. This is because a lower pKa corresponds to a higher tendency to donate a proton, indicating increased acidity.
How does resonance stabilization affect the acidity of carbonyl compounds?
-Resonance stabilization increases the acidity of carbonyl compounds by stabilizing the resulting anion. More resonance means more stability, which leads to a stronger acid, as the conjugate base is more stabilized.
Why are dicarbonyl compounds generally more acidic than monocarbonyl compounds?
-Dicarbonyl compounds are more acidic because the alpha position between two carbonyl groups can stabilize the anion through resonance with both carbonyls. This increased resonance stabilization makes the anion more stable and the compound more acidic.
What role does the alpha position play in the acidity of carbonyl compounds?
-The alpha position, adjacent to the carbonyl group, is acidic because when protonated, it can form an anion that is stabilized by resonance. This makes it easier for the compound to lose a proton, increasing its acidity.
Why are amides less acidic than esters and ketones?
-Amides are less acidic because nitrogen is less electronegative than oxygen and is less willing to donate electrons. This reduces the resonance stabilization at the alpha position, making amides less acidic compared to esters and ketones.
What is the general trend in acidity for monocarbonyl compounds like esters, ketones, and amides?
-In general, ketones are more acidic than esters, and esters are more acidic than amides. This trend is due to the difference in the resonance stabilization provided by oxygen (in esters and ketones) versus nitrogen (in amides).
Why is a diketone the most acidic among dicarbonyl compounds?
-A diketone is the most acidic because it has two carbonyl groups, allowing for the greatest resonance stabilization at the alpha position. There are no competing resonance effects, making the anion more stable and the compound more acidic.
How does the presence of an ester group affect the acidity of a carbonyl compound compared to a ketone?
-An ester group is less acidic than a ketone because the ester can engage in resonance, but its resonance is less effective than that of a ketone. The ester has an electron-donating alkyl group, which reduces the overall stabilization of the alpha position.
Why are dicarbonyl compounds with two ester groups less acidic than those with two ketone groups?
-Dicarbonyl compounds with two ester groups are less acidic because esters have more competing resonance effects, particularly from the alkyl group attached to the oxygen. This reduces the ability to stabilize the anion at the alpha position, making them less acidic than diketones.
What is the general rule for the acidity of monocarbonyl compounds versus dicarbonyl compounds?
-The general rule is that dicarbonyl compounds are more acidic than monocarbonyl compounds. The presence of two carbonyl groups leads to better resonance stabilization of the alpha position, making the anion more stable and the compound more acidic.
Outlines

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードMindmap

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードKeywords

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードHighlights

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードTranscripts

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレード5.0 / 5 (0 votes)