18.4 Solubility Equilibrium
Summary
TLDRThis video explores solubility equilibrium and the solubility product constant (Ksp). It explains how ionic compounds dissolve in water and reach an equilibrium where the ions recombine to form the compound. The solubility product constant is introduced as a key factor in determining whether a substance will precipitate out of a solution. The video also covers calculating Ksp values using experimental data and how the Ksp value can predict solubility and precipitation. The concept is demonstrated using examples such as silver chloride and barium carbonate, emphasizing the relationship between ion concentration and solubility in various solutions.
Takeaways
- 😀 Solubility equilibrium involves a reversible reaction where ionic compounds break into ions, but these ions can also recombine to form the compound again, creating an equilibrium.
- 😀 The equilibrium constant (Ksp) can be used to predict whether a substance will precipitate out of a solution, similar to how equilibrium constants are used in regular chemical reactions.
- 😀 A saturated solution contains the maximum amount of solute that can dissolve in a given amount of solvent. This does not mean it is necessarily concentrated, as solubility varies.
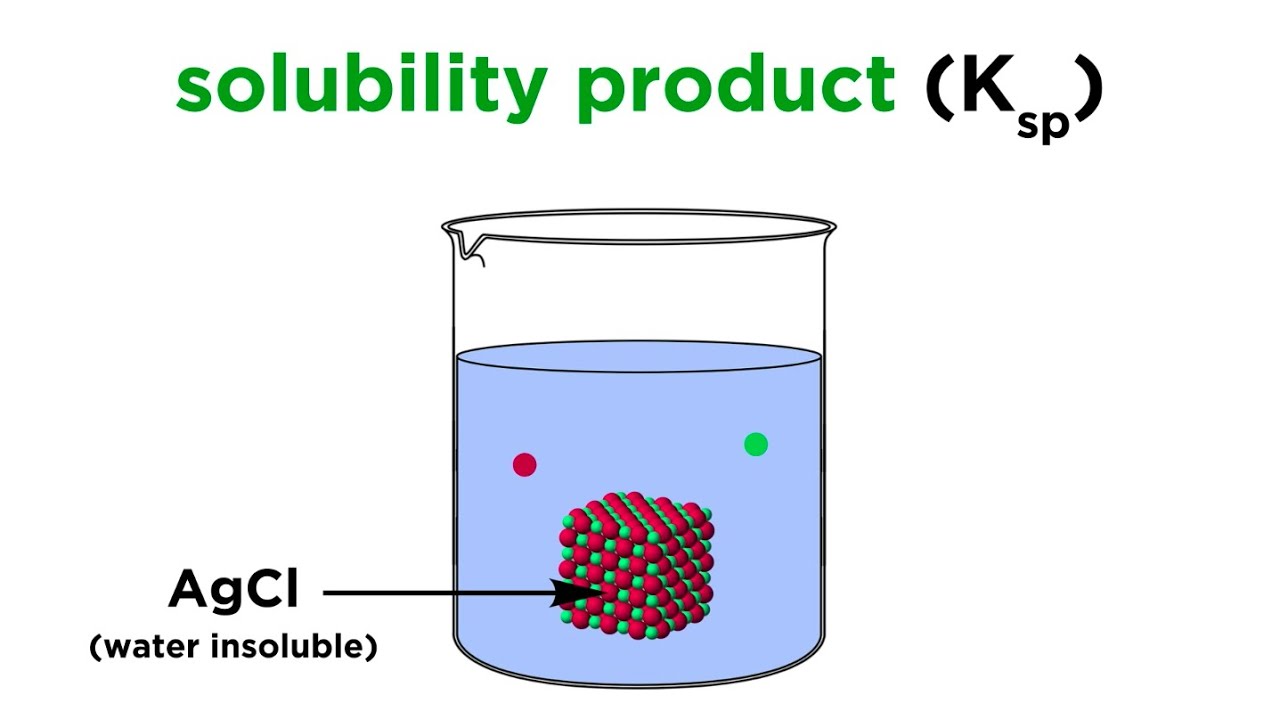
- 😀 Insoluble substances dissolve only a tiny amount in water (e.g., silver chloride), while slightly soluble substances dissolve in small quantities, and more soluble substances dissolve more easily.
- 😀 Silver chloride, being slightly soluble, reaches equilibrium quickly, with most of it precipitating out of the solution once the equilibrium is reached.
- 😀 The solubility product constant (Ksp) for a given compound can be determined experimentally. This helps predict the maximum concentration of ions in a saturated solution.
- 😀 The concentration of ions in a saturated solution can be calculated by first determining the solute's mass in solution and then converting that into molarity.
- 😀 For silver chloride, the Ksp value is calculated by multiplying the concentrations of the ions (Ag+ and Cl-) and squaring the result if there are multiple ions of the same type.
- 😀 Barium carbonate is another example of a slightly soluble compound. Its Ksp value helps calculate its solubility by setting up an equation for its ion concentrations.
- 😀 Precipitation can occur if the product of ion concentrations exceeds the Ksp value, which can happen when the solution is saturated or if temperature changes affect solubility.
- 😀 Ksp is especially useful for slightly soluble and insoluble compounds, but less reliable for highly soluble substances like salts and sugars due to their different dissolution behaviors.
Q & A
What is solubility equilibrium, and how does it relate to ionic compounds?
-Solubility equilibrium refers to the balance between the dissolution of an ionic compound into its constituent ions and the recombination of those ions back into the solid compound. This process is reversible, meaning the ions are constantly dissolving and recombining, reaching an equilibrium point where the rate of dissolution equals the rate of recombination.
What is the solubility product (Ksp), and how is it related to solubility equilibrium?
-The solubility product (Ksp) is a constant that represents the product of the concentrations of the ions in a saturated solution of a slightly soluble ionic compound, each raised to the power of its coefficient in the dissolution reaction. It helps predict whether a precipitate will form by comparing the ionic product to the Ksp.
What is the difference between a saturated solution and a concentrated solution?
-A saturated solution contains the maximum amount of solute that can dissolve in a solvent at a given temperature. A concentrated solution, however, has a high amount of solute, but it may not necessarily be saturated. For example, a concentrated solution may still have room for more solute to dissolve, depending on the solubility limit.
Why is silver chloride an example of a slightly soluble compound?
-Silver chloride is considered slightly soluble because only a small amount of it dissolves in water, and the rest precipitates out as a solid. This limited solubility leads to an equilibrium between the dissolved ions (Ag+ and Cl-) and the undissolved solid.
How is the Ksp value determined experimentally for a compound like silver chloride?
-The Ksp value for a compound like silver chloride is determined experimentally by finding the solubility of the compound in a solvent, then calculating the concentrations of the ions at equilibrium. Using these concentrations, the Ksp can be derived by multiplying the ion concentrations together.
How do you calculate the molarity of silver chloride when given the mass of solute and solvent?
-To calculate the molarity of silver chloride, first convert the mass of silver chloride to moles using its molar mass. Then, calculate the volume of the solvent in liters. Finally, divide the number of moles of silver chloride by the volume of the solvent to find the molarity.
What does the equation Ksp = [Ag+][Cl-] represent?
-This equation represents the solubility product for the dissolution of silver chloride in water, where [Ag+] is the concentration of silver ions, and [Cl-] is the concentration of chloride ions. The product of these concentrations at equilibrium gives the Ksp for silver chloride.
How does temperature affect the Ksp value and precipitation?
-Temperature influences the Ksp value because, in many cases, an increase in temperature can cause the Ksp to increase, allowing more solute to dissolve. If the temperature decreases, the Ksp generally decreases, which can cause the excess dissolved ions to precipitate out of the solution.
What is the relationship between ion product and Ksp when predicting precipitation?
-If the product of the ion concentrations (ion product) exceeds the Ksp value at a given temperature, precipitation occurs because the solution is supersaturated. If the ion product is less than the Ksp, the solution is unsaturated, and more solute can dissolve until equilibrium is reached.
How do precipitation reactions relate to the concept of solubility equilibrium?
-Precipitation reactions are a direct consequence of solubility equilibrium. When the concentration of ions in a solution exceeds the solubility limit (Ksp), the excess ions form a precipitate, shifting the equilibrium towards the solid phase to restore balance.
Outlines

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードMindmap

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードKeywords

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードHighlights

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードTranscripts

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレード関連動画をさらに表示

Introduction to Solubility equilibria
Solubility Product Constant (Ksp)

Kelarutan dan Hasil Kali Kelarutan (1) | Hubungan S dgn Ksp | Kimia Kelas 11

KELARUTAN DAN HASIL KALI KELARUTAN (Ksp) : KIMIA SMA KELAS 11

HASIL KALI KELARUTAN (KSP) -Simple Konsep- KIMIA 11 (Kursus Online Rp8.000 per BULAN :cek deskripsi)

Kelarutan dan Hasil Kali Kelarutan : Memprediksi Terbentuknya Endapan
5.0 / 5 (0 votes)
