Pengaruh Penambahan Suhu terhadap Pergeseran Kesetimbangan
Summary
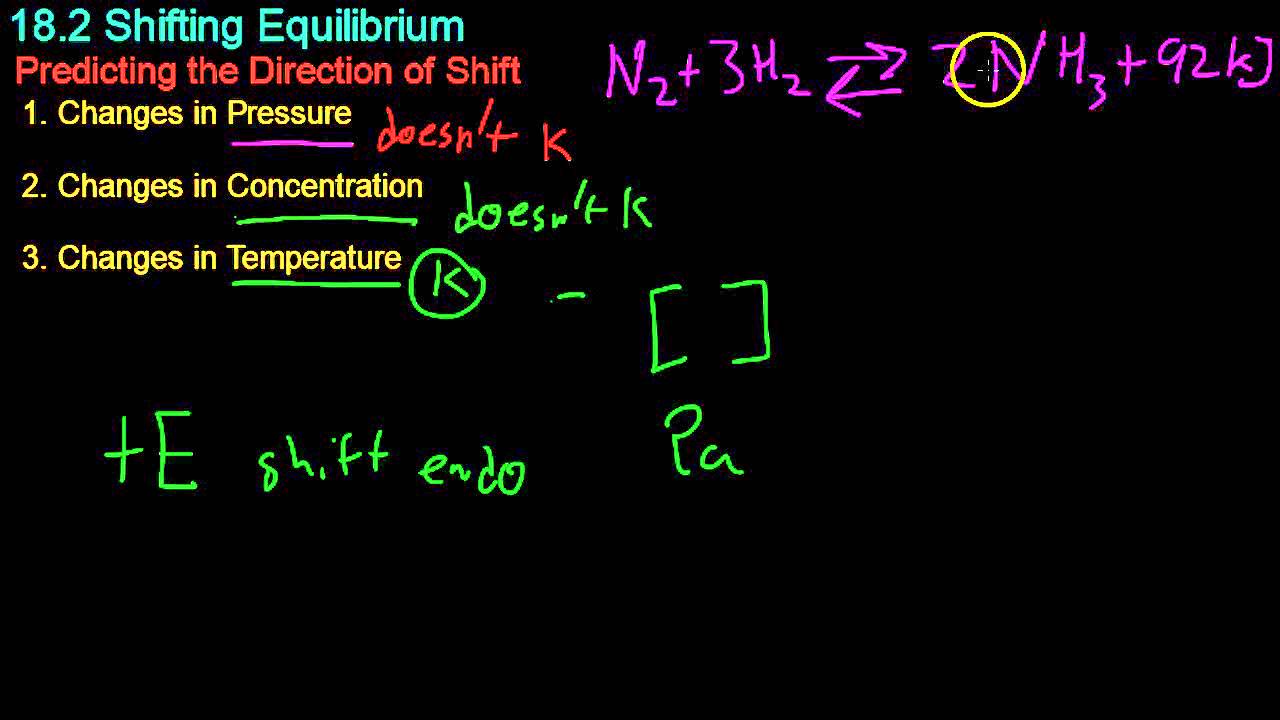
TLDRThe video explains how temperature affects the equilibrium of reactions, particularly focusing on an endothermic reaction involving complex ions. The experiment demonstrates that reducing temperature shifts the reaction towards the exothermic direction, with observable color changes. When energy is added (through heat), the reaction shifts towards the endothermic direction. Additionally, the video explores the behavior of nitrogen dioxide in equilibrium, showing that cooler temperatures cause the color to fade, while warmer temperatures make it more intense. The viewers are asked to analyze whether the nitrogen dioxide reaction is exothermic or endothermic.
Takeaways
- 😀 The script demonstrates the effect of temperature on equilibrium shifts in chemical reactions.
- 😀 The reaction used in the experiment involves a complex ion with cobalt, which is an endothermic reaction.
- 😀 Energy is added to the reactants to enable the reaction to proceed towards products, specifically in an endothermic reaction.
- 😀 Cold water reduces energy in the system, while hot water adds energy to the system.
- 😀 When the reactants are placed in cold water, the color changes from deep blue to purple, indicating a shift towards the reactants (left shift).
- 😀 When the reactants are placed in hot water, the color changes from red to purple, indicating a shift towards the products (right shift).
- 😀 If the energy (heat) is decreased, the reaction shifts toward the exothermic direction.
- 😀 If energy is added (by placing the system in hot water), the reaction shifts toward the endothermic direction.
- 😀 A similar experiment is conducted with nitrogen dioxide gas, where temperature also affects the color change in the gas.
- 😀 When the nitrogen dioxide gas system is placed in cold water, the color of the gas fades, indicating a shift in equilibrium.
- 😀 When the nitrogen dioxide gas system is placed in hot water, the color of the gas intensifies, indicating a shift towards the products in an endothermic reaction.
Q & A
What is the effect of temperature on equilibrium in a chemical reaction?
-Temperature changes can cause a shift in the equilibrium of a reaction. In general, increasing temperature shifts the equilibrium toward the endothermic direction, while decreasing temperature shifts it toward the exothermic direction.
What type of reaction is demonstrated in the experiment involving cobalt complex ions?
-The experiment demonstrates an endothermic reaction, where heat is absorbed. This is evidenced by the use of energy or heat being added to the reactants.
How does the addition of cold water affect the reaction involving cobalt complex ions?
-When the reaction is exposed to cold water, the heat energy is removed, causing the equilibrium to shift toward the exothermic direction. This results in a change in color from blue to purple.
What happens when the reaction with cobalt complex ions is exposed to hot water?
-When the reaction is exposed to hot water, heat energy is added, causing the equilibrium to shift toward the endothermic direction. This results in a color change from red to purple, moving toward the blue.
Why does the color of the cobalt complex ion change during the experiment?
-The color change occurs as the equilibrium of the reaction shifts. The color change from blue to purple (or red to purple) corresponds to a shift in the reaction based on the temperature change.
What does the shift in equilibrium indicate about the nature of the reaction?
-The shift in equilibrium indicates whether the reaction is exothermic or endothermic. A shift toward the exothermic direction suggests that heat is being released, while a shift toward the endothermic direction suggests that heat is being absorbed.
What role does energy play in shifting the equilibrium of a chemical reaction?
-Energy influences the direction of the equilibrium. Adding energy (heat) typically shifts the equilibrium toward the endothermic direction, while removing energy (cooling) shifts it toward the exothermic direction.
How does the experiment with nitrogen dioxide illustrate the effect of temperature on equilibrium?
-In the nitrogen dioxide experiment, when the system is exposed to cold water, the gas's color fades, indicating that the equilibrium has shifted. When exposed to hot water, the color becomes more intense, indicating a shift in the equilibrium toward the more concentrated side.
What is the expected color change in the nitrogen dioxide experiment when temperature is altered?
-When cooled, the color of the nitrogen dioxide gas becomes lighter or fades. When heated, the color becomes darker or more intense, indicating a shift in equilibrium due to temperature change.
How can the effect of temperature on chemical equilibrium be applied to real-world chemical processes?
-Understanding how temperature affects chemical equilibrium is crucial in industries such as chemical manufacturing, where reactions need to be controlled for maximum yield. Adjusting temperature can help optimize product formation by shifting equilibrium toward the desired products.
Outlines

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードMindmap

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードKeywords

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードHighlights

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードTranscripts

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレード関連動画をさらに表示
5.0 / 5 (0 votes)