Kimia Inti
Summary
TLDRThis video delves into nuclear chemistry, exploring the structure of atomic nuclei and the principles of nuclear reactions. Key topics include isotopes, isobars, isotones, nuclear stability, and radioactive decay. The speaker explains the concept of binding energy, how to calculate it, and the types of radioactive decay such as alpha, beta, and gamma radiation. Real-world examples and calculations are provided to demonstrate the practical applications of nuclear chemistry, making complex ideas accessible for students studying atomic theory and nuclear processes.
Takeaways
- 😀 Nuclear chemistry studies the structure of atomic nuclei and how it affects their stability, leading to processes like radioactive decay and nuclear transmutation.
- 😀 The atomic nucleus consists of protons and neutrons, which play a central role in determining the stability of the nucleus.
- 😀 Nuclear particles, including quarks, leptons (like electrons and neutrinos), and bosons (like photons and gluons), are key to understanding atomic and nuclear behavior.
- 😀 An isotope refers to atoms of the same element (same number of protons) with different numbers of neutrons, leading to variations in atomic mass.
- 😀 Isotones are different nuclei that have the same number of neutrons but different numbers of protons (different elements).
- 😀 Isobars are nuclei with the same atomic mass (number of protons + neutrons) but different atomic numbers, thus representing different elements.
- 😀 Stable nuclei, such as those of hydrogen, carbon, and oxygen, do not undergo radioactive decay and exist in nature without significant transformation over time.
- 😀 Radioactive nuclei can be classified as primary (naturally occurring), secondary (resulting from decay of primary radionuclides), or induced (created in laboratories).
- 😀 Binding energy of a nucleus refers to the energy required to separate its components (protons and neutrons). This energy is crucial for nuclear reactions and stability.
- 😀 Various types of radioactive decay include alpha decay, beta decay, gamma radiation, and neutron emission, each releasing specific particles or energy forms.
- 😀 Understanding nuclear reactions and decay processes is essential for applications in fields like medicine, energy, and environmental science.
Q & A
What is nuclear chemistry, and what does it study?
-Nuclear chemistry is the branch of chemistry that focuses on the study of atomic nuclei. It explores the structure of the nucleus, its stability, and the events that occur within the nucleus, such as radioactivity and nuclear transmutation.
What are the main components of an atom that nuclear chemistry focuses on?
-Nuclear chemistry primarily focuses on the nucleus of the atom, which consists of protons and neutrons. Electrons are considered secondary in this context as they revolve around the nucleus.
What is the difference between isotopes, isobars, and isotones?
-Isotopes are atoms of the same element with the same number of protons but different numbers of neutrons. Isobars are atoms with the same mass number but different atomic numbers. Isotones are atoms with the same number of neutrons but different atomic numbers.
How do protons and neutrons contribute to the stability of the nucleus?
-Protons and neutrons are the nucleons that make up the nucleus. Their number and arrangement influence the stability of the nucleus. An imbalance in the number of protons and neutrons can lead to radioactive decay.
What is the significance of binding energy in nuclear chemistry?
-Binding energy is the energy required to separate a nucleus into its individual nucleons. It indicates the stability of the nucleus, with higher binding energy implying a more stable nucleus.
What are the four main types of nuclear decay?
-The four main types of nuclear decay are alpha decay (α), beta decay (β), gamma decay (γ), and neutron emission. Each type involves the emission of specific particles or energy from the nucleus.
What is the formula for calculating binding energy in nuclear reactions?
-The formula for calculating binding energy is E_b = Δm × 931.5 MeV, where Δm is the mass defect (the difference between the mass of the nucleons and the mass of the nucleus).
What are radionuclides, and how are they categorized?
-Radionuclides are unstable nuclei that undergo radioactive decay. They are categorized into primary radionuclides (naturally occurring and stable for long periods), secondary radionuclides (formed from the decay of primary radionuclides), and induced radionuclides (produced artificially in laboratories).
How does the concept of isotopic decay help in determining the age of substances?
-Isotopic decay, particularly using isotopes with known half-lives (like Carbon-14), allows scientists to estimate the age of substances by measuring the remaining amount of the isotope and calculating how long it has been decaying.
What role do neutrons play in nuclear reactions, and how are they used in creating new elements?
-Neutrons are essential in nuclear reactions, particularly in nuclear fission. They can be used to induce reactions by colliding with atomic nuclei, resulting in the creation of new elements or isotopes, as seen in nuclear reactors or artificial transmutations in laboratories.
Outlines

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードMindmap

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードKeywords

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードHighlights

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードTranscripts

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレード関連動画をさらに表示
Nuclear Chemistry Part 2 - Fusion and Fission: Crash Course Chemistry #39

Tudo se transforma, Energia e Impacto Ambiental, Energia Nuclear 1

NUCLEAR - GCSE Physics (AQA Topic P4 & Other Boards)

Fisika kelas 12 | Fisika Inti dan Radioaktivitas
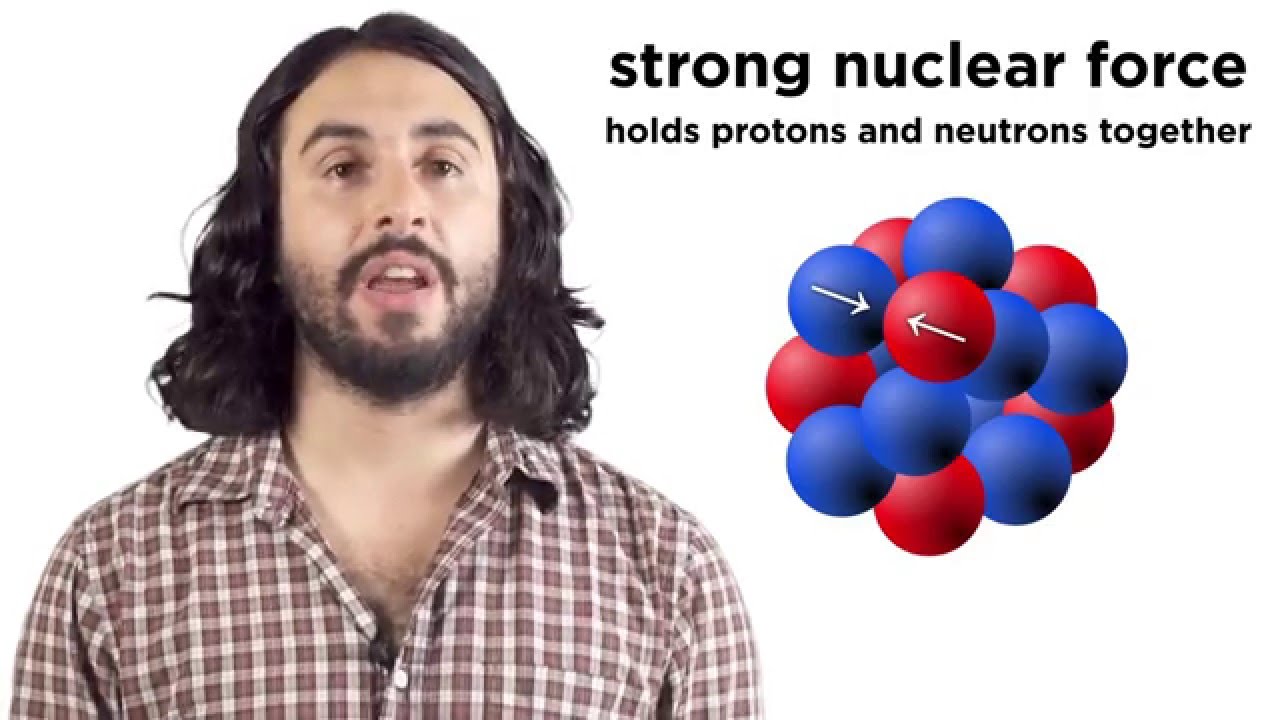
Nuclear Reactions, Radioactivity, Fission and Fusion

Instrumen NMR | Prinsip Kerja NMR
5.0 / 5 (0 votes)
