Amino Acids and Zwitterions (A-level Chemistry)
Summary
TLDRThis video explains the properties of amino acids, focusing on their ability to form zwitterions—molecules with both positive and negative charges but no overall charge. It covers how amino acids, with an amine and a carboxyl group, can exist in different forms depending on pH: as positively charged ions in acidic solutions, zwitterions in neutral solutions, and negatively charged ions in alkaline solutions. The video also touches on optical activity, with amino acids being optically active due to their chiral centers, and highlights the concept of the isoelectric point, which varies for different amino acids.
Takeaways
- 😀 Amino acids have both amine (NH2) and carboxyl (COOH) groups, giving them acidic and basic properties.
- 😀 Amino acids can form zwitter ions, which have both positive and negative charges but no overall charge.
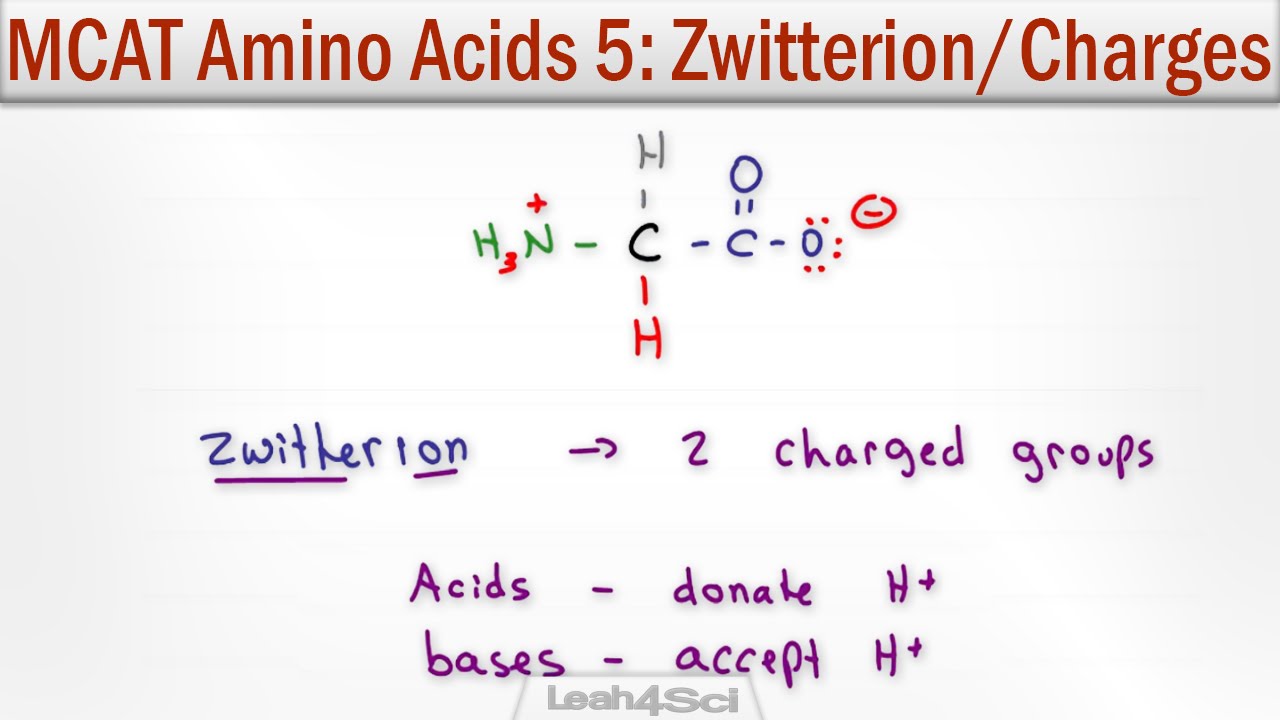
- 😀 A zwitter ion forms through an internal acid-base reaction where the carboxyl group loses an H+ ion and the amine group accepts it.
- 😀 The pH of a solution affects the ionic form of amino acids: in acidic solutions, they become positively charged, and in alkaline solutions, negatively charged.
- 😀 The isoelectric point (pI) is the pH at which an amino acid exists as a zwitter ion with no overall charge.
- 😀 Amino acids can exist in different charged forms depending on the pH of the solution, such as positively charged in acidic solutions and negatively charged in alkaline solutions.
- 😀 The strength of the carboxyl group and the basicity of the amine group vary based on the amino acid’s R group, influencing the pH at which zwitter ions form.
- 😀 Naturally occurring amino acids are optically active due to their chiral center (the central carbon bonded to four different groups).
- 😀 Optical isomerism in amino acids results in two enantiomers that rotate plane polarized light in opposite directions.
- 😀 The basic structure of amino acids includes an NH2 group, a COOH group, and an R group, with glycine being the simplest amino acid, where the R group is just a hydrogen atom.
Q & A
What are amino acids, and what groups do they contain?
-Amino acids are organic compounds that contain both an amine group (NH2) and a carboxyl group (COOH). These groups give them their acidic and basic properties, making them essential for forming proteins.
What is the role of the R group in amino acids?
-The R group (side chain) in amino acids is unique to each amino acid and determines its properties, such as polarity, charge, and size. It is attached to the central alpha carbon.
What is a zwitter ion?
-A zwitter ion is a molecule that has both a positive and a negative charge in its structure but no overall charge. This occurs when an amino acid forms an internal acid-base reaction between its amine and carboxyl groups.
How does pH affect the charge of an amino acid?
-The charge of an amino acid changes with pH. In acidic solutions, the carboxylate group accepts a proton and becomes neutral, while the amine group remains positively charged. In alkaline solutions, the amine group loses a proton, making the molecule negatively charged.
What is the isoelectric point of an amino acid?
-The isoelectric point is the pH at which an amino acid exists as a zwitter ion, with no overall charge. This pH varies depending on the amino acid and its R group.
How do the acidic and basic properties of amino acids relate to their zwitter ion formation?
-Amino acids have both acidic (carboxyl) and basic (amine) properties, allowing them to form zwitter ions. In neutral pH solutions, the carboxyl group loses a proton, while the amine group accepts one, balancing the positive and negative charges.
How do different R groups influence the formation of zwitter ions?
-The strength of the carboxyl and amine groups in an amino acid is affected by the R group. A more acidic R group will make the carboxylate group more likely to accept a proton at a lower pH, while a stronger basic R group will affect how easily the amine group accepts a proton.
What happens when an amino acid is placed in a highly acidic solution?
-In a highly acidic solution, the carboxylate group of a zwitter ion will accept a proton, forming a neutral carboxyl group, while the amine group remains positively charged, making the amino acid a positively charged ion.
What is optical isomerism, and how does it apply to amino acids?
-Optical isomerism occurs when a compound has a chiral center, meaning it can exist in two mirror-image forms (enantiomers) that rotate plane-polarized light in opposite directions. Most amino acids (except glycine) exhibit optical isomerism due to the chiral center at their alpha carbon.
Why do naturally occurring amino acids rotate plane-polarized light?
-Naturally occurring amino acids rotate plane-polarized light because they are chiral. The central alpha carbon has four different groups attached, making it asymmetric and allowing the molecule to rotate light in different directions depending on the isomer.
Outlines

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードMindmap

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードKeywords

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードHighlights

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードTranscripts

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレード関連動画をさらに表示

AMINOSÄUREN (Aufbau, Eigenschaften, Zwitterion) | Chemie Tutorial
Zwitterion and Amino Acid Charge Given pH and pKa

Hydrogen Bonds - What Are Hydrogen Bonds - How Do Hydrogen Bonds Form
2-7 What affects the solubility of a substance in water? (Cambridge AS & A Level Biology)

The Chemistry of Water Screencast Session 1

Electrophoresis of amino acids
5.0 / 5 (0 votes)
