10.4 Changes of State (2/2)
Summary
TLDRThis video explains the phase changes of freezing, melting, and their associated energy dynamics. It details how freezing releases energy, converting a liquid into a solid, and how adding energy can reverse this, turning a solid back into a liquid. The concept of molar enthalpy of fusion is introduced to explain energy absorption during melting. A phase diagram for water is also discussed, highlighting key points like the triple point, where solid, liquid, and gas phases coexist, and the critical point, where water can no longer exist as a liquid. This exploration covers fundamental thermodynamics principles in phase changes.
Takeaways
- 😀 Freezing is the phase change from a liquid to a solid, releasing energy as heat.
- 😀 According to the law of conservation of energy, energy lost during freezing must be accounted for in the reaction.
- 😀 Freezing occurs at a specific freezing point, which is the temperature at normal atmospheric pressure (1 atm or 101.3 kPa).
- 😀 At the freezing point, the temperature of both the solid and liquid is the same, but the energy released is due to intermolecular forces.
- 😀 Melting is the reverse process of freezing, and it can be achieved by adding energy (e.g., heating with hands).
- 😀 Freezing and melting are reversible processes and can reach an equilibrium similar to condensation and evaporation.
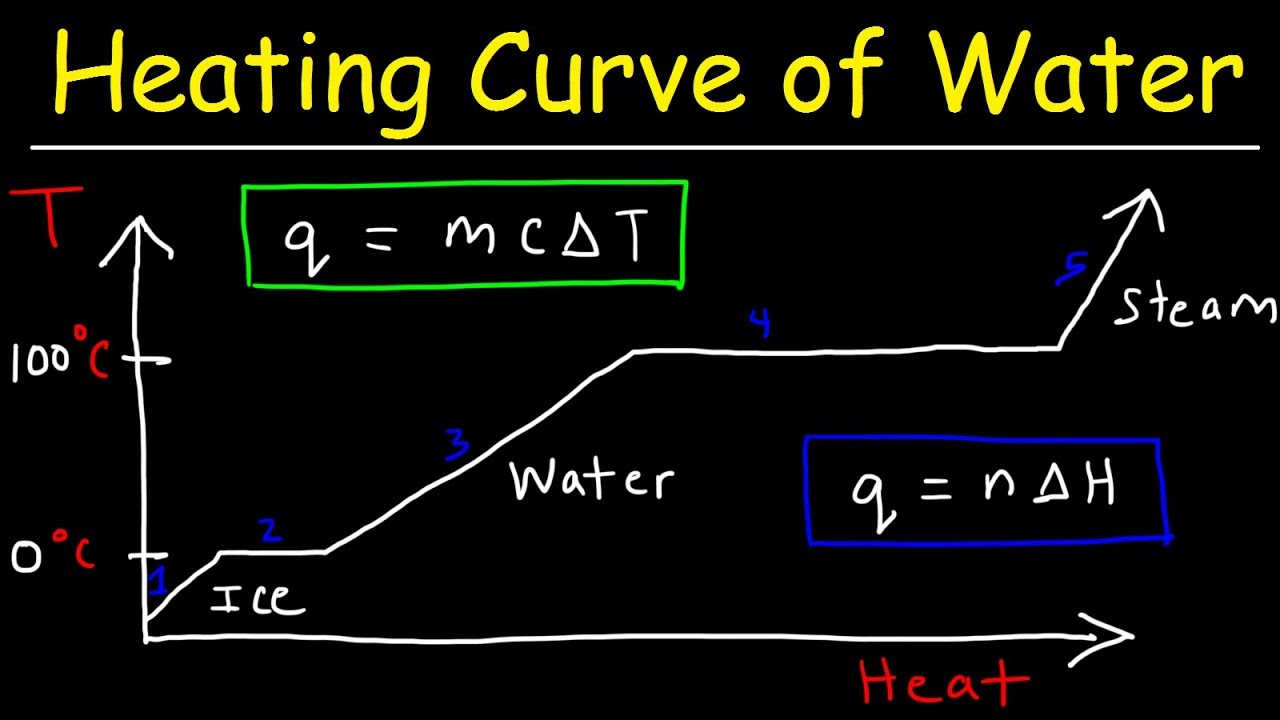
- 😀 During freezing or melting, the temperature of the substance stays constant at the freezing/melting point until the phase change is complete.
- 😀 The molar enthalpy of fusion is the energy required to convert 1 mole of a substance from solid to liquid at the freezing/melting point.
- 😀 The energy added during melting increases potential energy between molecules, not kinetic energy, so temperature does not increase during this phase change.
- 😀 Phase diagrams show the phase changes of a material at different temperatures and pressures, helping to visualize conditions for freezing, melting, boiling, and condensation.
- 😀 The triple point on a phase diagram is where the solid, liquid, and gas states of a substance coexist, and the critical point marks the temperature and pressure beyond which a substance cannot exist as a liquid.
Q & A
What is the difference between freezing and melting?
-Freezing is the process of a liquid turning into a solid, releasing energy in the form of heat, while melting is the opposite process, where a solid turns into a liquid by absorbing energy.
Why does energy release during freezing?
-Energy is released during freezing because the molecules in the liquid state lose potential energy as they form bonds to become solid, and this energy is released as heat to the environment.
What is the freezing point, and why is it significant?
-The freezing point is the temperature at which a liquid turns into a solid under normal atmospheric pressure. It is significant because it represents the temperature at which the phase change between liquid and solid can occur.
How does the law of conservation of energy apply to freezing and melting?
-According to the law of conservation of energy, energy cannot be destroyed; it is transferred or converted. In freezing, energy is released to the surroundings, and in melting, energy is absorbed from the environment, both without changing the system’s total energy.
What is the molar enthalpy of fusion?
-The molar enthalpy of fusion is the amount of heat energy required to melt one mole of a solid into a liquid at its melting point. This energy increases the potential energy between molecules but does not affect the temperature.
Why does the temperature stay constant during the phase change?
-During phase changes like melting or freezing, the temperature remains constant because the added or released energy goes into changing the state of the substance rather than raising or lowering the temperature.
What happens to the temperature of water when ice melts in a glass of water?
-When ice melts in a glass of water, the temperature of the water remains constant at 0°C until all the ice has melted. Only after the ice has fully transitioned to liquid does the temperature of the water begin to rise.
What is a phase diagram and what does it show?
-A phase diagram is a graphical representation that shows the states (solid, liquid, gas) of a substance at different temperatures and pressures, as well as the conditions under which phase changes (freezing, melting, boiling) occur.
What is the triple point on a phase diagram?
-The triple point is the unique combination of temperature and pressure at which a substance can coexist in all three phases: solid, liquid, and gas. For water, this occurs at 0.06 atm and 0.01°C.
What is the critical point in the phase diagram of water?
-The critical point is the temperature and pressure beyond which water can no longer exist as a liquid. For water, this occurs at 374°C and 225 atm, where the molecules have too much energy to remain in liquid form.
Outlines

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードMindmap

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードKeywords

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードHighlights

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードTranscripts

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレード関連動画をさらに表示

SCIENCE 7: Quarter 1-LC 5: PHASE CHANGES OF THE STATES OF MATTER| MATATAG CURRICULUM

Heating Curves and Cooling Curves

KALOR DAN PERUBAHAN WUJUD | KALOR DAN PERPINDAHAN KALOR
Heating Curve and Cooling Curve of Water - Enthalpy of Fusion & Vaporization

Science 8 - Quarter 3 Week 3-4 | Phase Changes

The Four States of Matter - Explained
5.0 / 5 (0 votes)
