The difference between osmosis and diffusion
Summary
TLDRThis video script explains the key principles of osmosis and diffusion in the human body. It describes how osmosis causes water to move across membranes in response to solute concentration, potentially leading to cell swelling or rupture. The process of diffusion is also explored, where solutes move from high to low concentration across permeable membranes. The video outlines how the body’s compartments—intracellular and extracellular—are separated by membranes that regulate the movement of water, solutes, and proteins. Emphasizing the role of kidneys in managing body fluid concentration, it highlights the importance of osmotic balance in maintaining bodily functions.
Takeaways
- 😀 Osmosis involves the movement of water through a semi-permeable membrane from low to high solute concentration, aiming for equilibrium between compartments.
- 😀 Diffusion refers to the movement of solute from high to low concentration across a permeable membrane until equilibrium is reached, without water movement.
- 😀 The body is divided into intracellular (inside cells) and extracellular (outside cells) compartments, separated by selective membranes.
- 😀 The cell membrane is permeable to water and small non-polar molecules but impermeable to charged particles like sodium and potassium, which require transport mechanisms.
- 😀 In osmosis, water moves to dilute a high solute concentration, potentially causing cells (like red blood cells) to swell and rupture (hemolysis) if exposed to hypotonic plasma.
- 😀 In diffusion, solute moves across the membrane without affecting water distribution, as the membrane is permeable to the solute.
- 😀 The capillary wall separates the interstitial fluid and plasma, allowing water and small molecules to cross but restricting large proteins like albumin and cells.
- 😀 The cerebrospinal fluid (CSF) maintains the same osmolality as plasma, preventing water shifts that could imbalance this relationship.
- 😀 The kidneys have specialized epithelial cells in the GU tract that are impermeable to water unless unlocked by ADH, enabling regulation of body fluid concentrations.
- 😀 Osmolality equilibrium is crucial across the body; differences in compartment sizes are determined by the number of osmoles in each space, such as plasma and interstitial fluid.
Q & A
What is the primary difference between the forces we experience in daily life and the forces governing fluid movement in the body?
-While we are familiar with gravity and inertia, which govern how we move, fluid movement in the body is controlled by processes like osmosis and diffusion, which operate differently from mechanical forces.
How does osmosis work in the experiment with the semi-permeable membrane?
-In the experiment, when solute is added to one side of the beaker, it increases the osmotic pressure. This draws water from the other side of the membrane toward the higher solute concentration, balancing the concentration but increasing the volume on that side.
What can happen to red blood cells when plasma becomes hypotonic?
-When plasma becomes hypotonic, water rushes into red blood cells, causing them to swell. If this process continues, the cells can rupture, leading to hemolysis.
What distinguishes osmosis from diffusion in terms of the type of movement across membranes?
-Osmosis involves the movement of water from low to high solute concentration across a semi-permeable membrane, while diffusion refers to the movement of solute from high to low concentration across a permeable membrane.
What is the key feature of the membrane in the diffusion experiment?
-The membrane in the diffusion experiment is permeable to the solute, allowing it to move freely from areas of higher concentration to lower concentration until equilibrium is reached.
Why are the cell and capillary membranes permeable to water but not to solutes like sodium or potassium?
-The cell membrane is permeable to water and small non-polar molecules like urea and CO2, but impermeable to charged particles like sodium and potassium. These ions require specific transport mechanisms, such as sodium-potassium pumps, to move across the membrane.
What role does the capillary wall play in regulating fluid movement?
-The capillary wall separates the interstitial fluid from the plasma. It is permeable to water and small molecules, but restricts the passage of larger proteins and cells, such as red blood cells and platelets.
How does the body maintain osmotic balance across compartments like plasma, CSF, and interstitial fluid?
-Water moves freely across all compartments, maintaining equal osmolality throughout the body. This balance is crucial for preventing drastic shifts in water between compartments, which could disrupt bodily functions.
What makes the kidney unique in terms of water permeability compared to other body compartments?
-The kidney, specifically the GU tract, is unique because water permeability is regulated by antidiuretic hormone (ADH). This allows the kidney to create concentration gradients and excrete fluids with varying osmotic concentrations.
What are the two main compartments of the body, and how are they separated?
-The body is divided into two main compartments: the intracellular compartment (inside cells) and the extracellular compartment (outside cells). These are separated by the cell membrane, which controls what passes into and out of cells.
Outlines

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードMindmap

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードKeywords

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードHighlights

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードTranscripts

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレード関連動画をさらに表示

2025 ATI TEAS 7 Science Chemistry with Nurse Cheung | Properties of Solutions, Osmosis, Diffusion

Transport in Cells: Diffusion and Osmosis | Cells | Biology | FuseSchool
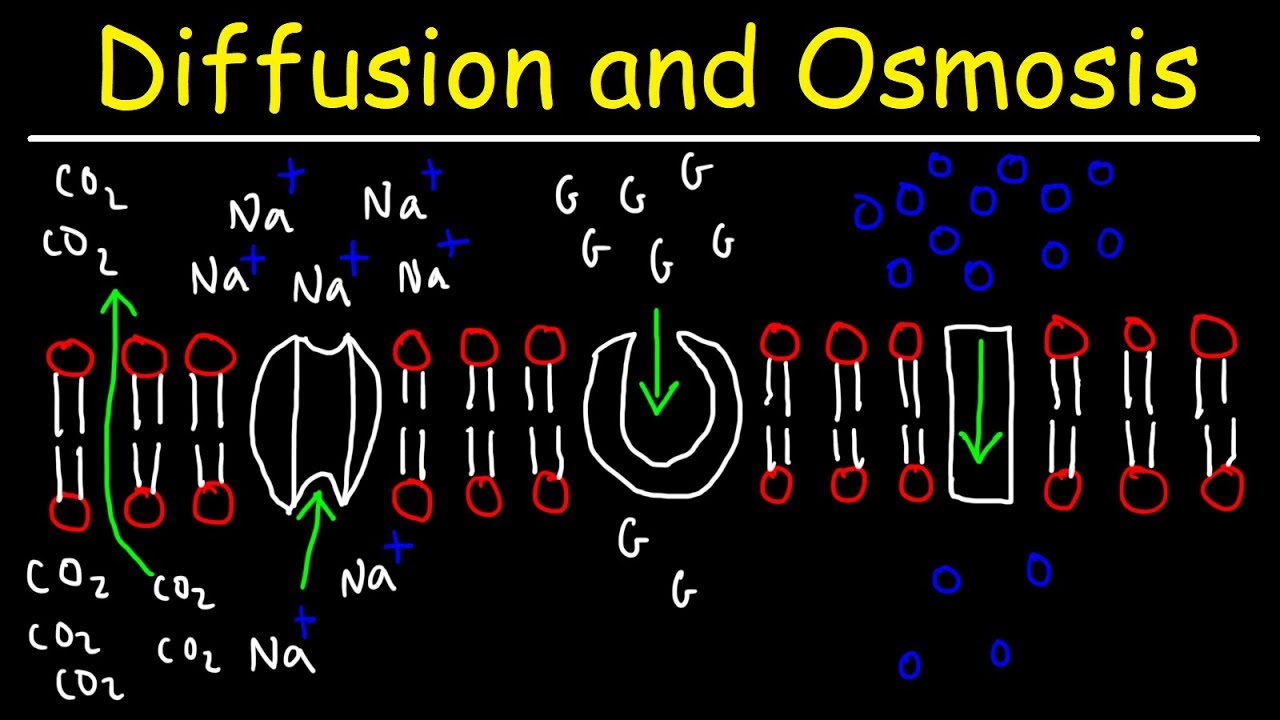
Diffusion and Osmosis - Passive and Active Transport With Facilitated Diffusion

Cairan dan Elektrolit - part 1
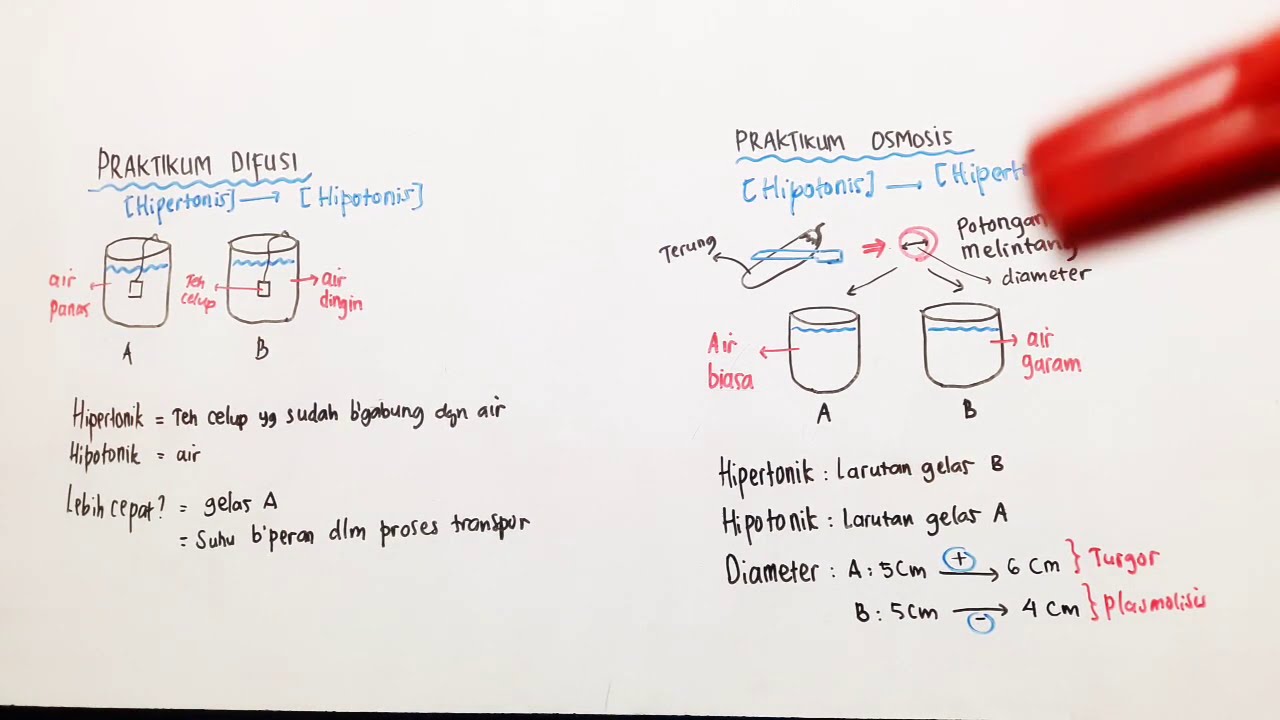
Praktikum Osmosis dan Difusi

Cell Membrane Transport (Passive & Active) Diffusion, Osmosis, Hydrostatic Oncotic Pressure Colloid
5.0 / 5 (0 votes)
