MOLARIDAD (M), molalidad (m) y partes por millón (ppm)
Summary
TLDRIn this final video of a three-part series, the focus is on calculating the concentration of solutions using molarity (M), molality (m), and parts per million (ppm). The tutorial walks through key formulas and practical exercises, such as calculating molarity from moles and volume, molality from moles and solvent mass, and ppm from milligrams of solute and solution mass. The video reinforces the importance of proper unit conversions, ensuring that viewers can confidently apply these calculations to various scenarios. This engaging lesson wraps up with a reminder to subscribe, like, and comment for feedback.
Takeaways
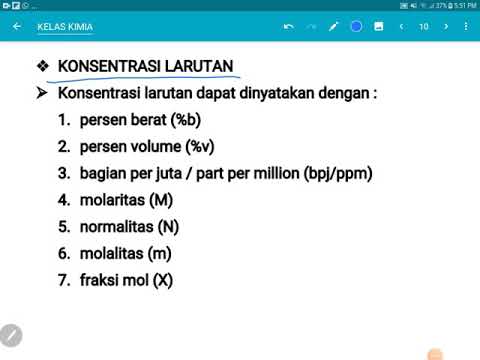
- 😀 Molarity (M) is the number of moles of solute per liter of solution. Formula: M = moles of solute / liters of solution.
- 😀 To calculate molarity, ensure that the volume of the solution is in liters. If it's in milliliters, convert it to liters first.
- 😀 Molality (m) is the number of moles of solute per kilogram of solvent. Formula: m = moles of solute / kilograms of solvent.
- 😀 For molality, make sure that the solvent mass is in kilograms, and the solute is in moles.
- 😀 Parts per million (ppm) is used for very dilute solutions. Formula: ppm = milligrams of solute / kilograms of solution.
- 😀 When calculating ppm, convert grams to milligrams if necessary, and ensure the solution mass is in kilograms.
- 😀 In the first example, converting 2500 mL of solution to 2.5 L is necessary for the molarity calculation.
- 😀 When working with moles of a substance, convert the given mass in grams to moles using the molar mass (e.g., 124 grams of sodium oxide equals 2 moles).
- 😀 To convert from grams to moles, use the molar mass of the compound, which can be found using the periodic table.
- 😀 Always ensure that the units of the solute and solvent match the required units for the specific concentration formula being used.
- 😀 When solving problems, break down each step, such as unit conversions, to ensure the calculations are accurate and follow the correct formulas.
Q & A
What is molarity and how is it calculated?
-Molarity is the concentration of a solution expressed as moles of solute per liter of solution. It is calculated using the formula: M = moles of solute / liters of solution.
What is the significance of converting solution volume to liters in molarity calculations?
-It is crucial to convert the solution volume to liters, as molarity is defined in terms of liters. Without this conversion, the result would not be correct.
How do you convert milliliters to liters?
-To convert milliliters to liters, divide the number of milliliters by 1000. For example, 2500 milliliters equals 2.5 liters.
What is the formula for molality?
-Molality (m) is calculated using the formula: m = moles of solute / kilograms of solvent. Molality is different from molarity because it uses the mass of the solvent rather than the volume of the solution.
What do you do if the solute is given in grams instead of moles for molarity calculations?
-If the solute is given in grams, you must convert it to moles by using the molar mass of the solute before applying the molarity formula.
How do you calculate molarity from a given mass of solute?
-To calculate molarity from a mass of solute, first convert the mass to moles using the molar mass of the solute, then divide by the volume of the solution in liters.
What is the formula for calculating parts per million (ppm)?
-The formula for calculating parts per million (ppm) is: ppm = (milligrams of solute / kilograms of solution). It expresses the concentration of a solute in terms of milligrams per kilogram.
What do you need to do if the solute is in a unit other than milligrams for ppm calculations?
-If the solute is in a unit other than milligrams (e.g., grams), you must convert it to milligrams before using the ppm formula.
What is the main difference between molarity and molality?
-The main difference is that molarity is based on the volume of the solution, while molality is based on the mass of the solvent. Molality is particularly useful when the solution's temperature changes because volume can expand or contract with temperature, while mass remains constant.
In the video, what was the first step in solving the example problems related to molarity?
-The first step in solving the molarity problem was to convert the solution volume from milliliters to liters, as the molarity formula requires the volume to be in liters.
Outlines

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードMindmap

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードKeywords

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードHighlights

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードTranscripts

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレード関連動画をさらに表示
Kelas Kimia : Konsentrasi Larutan (% berat, % volume, ppm / bpj)

Unit 8 Solutions Concept 2 Notes

Aula 7 - Concentração das soluções (Partes por milhão, ppm e Partes por bilhão, ppb)

cara menghitung Konsentrasi ZPT

Trick to Calculate Molarity | Molarity practice problems

Difference between Molarity and Molality
5.0 / 5 (0 votes)
