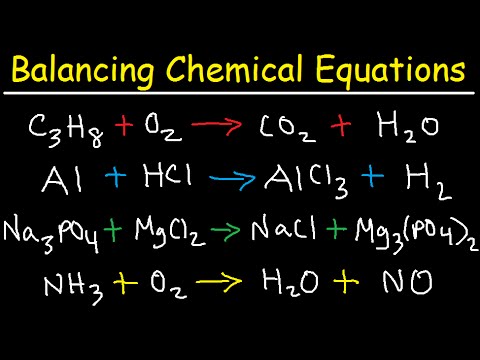
Balancing Chemical Equations Practice Problems
Summary
TLDRThis video provides a comprehensive guide to balancing chemical equations, starting with simple examples and progressing to more complex scenarios. It emphasizes the importance of using coefficients rather than altering subscripts to maintain the integrity of compounds. Viewers learn step-by-step techniques to balance equations involving various elements, including xenon, silver, potassium, and more. The tutorial highlights common misconceptions and demonstrates the systematic approach needed to achieve balanced equations, making it an invaluable resource for students and anyone looking to understand chemical reactions better.
Takeaways
- 😀 Balancing chemical equations involves ensuring the same number of atoms for each element on both sides of the equation.
- 📝 Coefficients (numbers placed in front of compounds) can be adjusted to balance the equation, while subscripts (numbers within the chemical formulas) cannot be changed.
- 🔍 When balancing equations, create a chart to track the number of atoms for each element on both sides.
- ⚖️ If elements balance on one side but not the other, add coefficients to the compounds on the side that has fewer atoms.
- 🌡️ When dealing with multiple elements, start with those that appear in fewer compounds for easier balancing.
- 🔄 Always check how adding a coefficient affects other elements in the equation to ensure overall balance.
- 🔢 For equations with parentheses, remember that everything inside them is multiplied by the coefficient placed in front.
- 🧪 More complex equations often require multiple steps and careful tracking of all elements involved.
- 💡 When balancing polyatomic ions, treat them as a single unit when they appear on both sides of the equation.
- 📊 Practice is essential for mastering the balancing of chemical equations, as complexity increases with each example.
Q & A
What is the first step in balancing a chemical equation?
-The first step is to count the number of atoms of each element on both sides of the equation and create a chart to keep track.
Why can't you change the subscripts in a chemical equation?
-You cannot change the subscripts because they represent the fixed number of atoms in a molecule; only coefficients can be adjusted to balance the equation.
How do coefficients affect the number of atoms in a chemical equation?
-Coefficients multiply the number of atoms in the compounds they precede, altering the total count of atoms in the equation.
In the equation involving xenon and fluorine, what was done to balance the fluorine atoms?
-To balance the fluorine atoms, a coefficient of 3 was added in front of F2 on the reactant side to match the 6 fluorine atoms on the product side.
What elements were involved in the second example with silver and hydrogen?
-The elements involved were silver (Ag), hydrogen (H), and sulfur (S), where the silver atoms were balanced by adding a coefficient of 2 in front of Ag.
When balancing the equation with potassium, how was the oxygen accounted for?
-Oxygen was initially left for later balancing, and the potassium was balanced first, followed by adjusting coefficients to ensure all elements matched.
What strategy was used to handle the complex equation with parentheses?
-The strategy involved balancing the elements inside the parentheses first by applying the coefficient to the entire compound before addressing the individual elements.
Why is it important to balance more complex elements first?
-Balancing more complex elements first simplifies the process, as it reduces the number of variables to adjust later, making it easier to achieve a balanced equation.
How was the final balance achieved in the aluminum and hydrogen equation?
-The final balance was achieved by first balancing sulfur and oxygen, then adjusting hydrogen and finally aluminum to ensure all elements had equal counts on both sides.
What is a common misconception when balancing chemical equations?
-A common misconception is that you can change subscripts to balance an equation; however, this is incorrect as it alters the fundamental composition of the compounds.
Outlines

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードMindmap

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードKeywords

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードHighlights

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードTranscripts

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレード5.0 / 5 (0 votes)