ELECTROPHILE VS NUCLEOPHILE : 🧪 Comment les différencier en REACTIVITE CHIMIQUE ?
Summary
TLDRIn this informative video, the presenter breaks down the distinctions between nucleophiles and electrophiles, simplifying complex concepts for better understanding. Nucleophiles, rich in electrons, engage in chemical reactions by attacking electron-deficient sites, while electrophiles, typically positively charged or electron-poor, seek out areas with high electron density. The video emphasizes key characteristics and examples of both types, including Lewis bases and acids, as well as radical reactions. Through clear explanations and relatable examples, the presenter aims to clarify these fundamental concepts in chemistry, setting the stage for further exploration of chemical activities.
Takeaways
- 😀 Understanding the difference between nucleophiles and electrophiles is crucial in chemistry.
- 🔍 Nucleophiles are electron-rich species that donate electrons to form bonds.
- ⚡ Electrophiles are electron-poor species that accept electrons during chemical reactions.
- 💡 Nucleophiles can be substances like alkoxides, hydroxides, and carbanions.
- 🌟 Common examples of nucleophiles include alcohols and certain halides.
- 🌀 Electrophiles often have a central atom that is electron-deficient and can attract electrons.
- 🔗 An example of an electrophile is a positively charged cation, such as CH3+.
- 📊 Electrophiles are typically characterized by their positive charge or electron deficiency.
- 🧪 The terms nucleophile and electrophile are often tested in chemistry quizzes and exams.
- 👨🏫 The video promises to cover more complex chemical concepts in future videos.
Q & A
What is the main focus of the video?
-The video focuses on explaining the differences between nucleophiles and electrophiles in chemistry.
What are nucleophiles characterized by?
-Nucleophiles are characterized as electron-rich species that can donate electron pairs to form new bonds.
Can you provide examples of nucleophiles mentioned in the video?
-Examples of nucleophiles include hydroxide ions (OH⁻), alcohols, and certain carbanions.
How do nucleophiles interact in chemical reactions?
-Nucleophiles typically attack sites with low electron density, forming new bonds through their electron donation.
What defines electrophiles?
-Electrophiles are defined as electron-poor species that are attracted to regions of high electron density and can accept electrons.
What types of species are considered electrophiles?
-Electrophiles include cations (such as CH₃⁺) and Lewis acids, which can accept electron pairs.
What is the significance of electron density in distinguishing nucleophiles and electrophiles?
-Nucleophiles are electron-rich, while electrophiles are electron-poor, making their interactions in chemical reactions dependent on these properties.
What role do Lewis acids play in the context of electrophiles?
-Lewis acids are considered electrophiles because they can accept electron pairs from nucleophiles during reactions.
What will the speaker cover in future videos related to this topic?
-The speaker will cover more advanced topics such as additions, substitutions, and eliminations in chemical reactions.
Why is understanding nucleophiles and electrophiles important in chemistry?
-Understanding these concepts is essential for grasping various chemical reactions and their mechanisms.
Outlines

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードMindmap

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードKeywords

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードHighlights

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードTranscripts

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレード関連動画をさらに表示

Nucleophiles and Electrophiles: Crash Course Organic Chemistry #12

Software Testing Tutorial #12 - What is Unit Testing/Component Testing

Negligência, Imprudência e Imperícia | Culpa Consciente x Dolo Eventual | Direito Penal - PARTE VIII

LOGARITMA ITU GAMPANG!! Bahas Logaritma Kelas 10 | Study With Jerome Polin

Draw On Liquidity (only video you need)
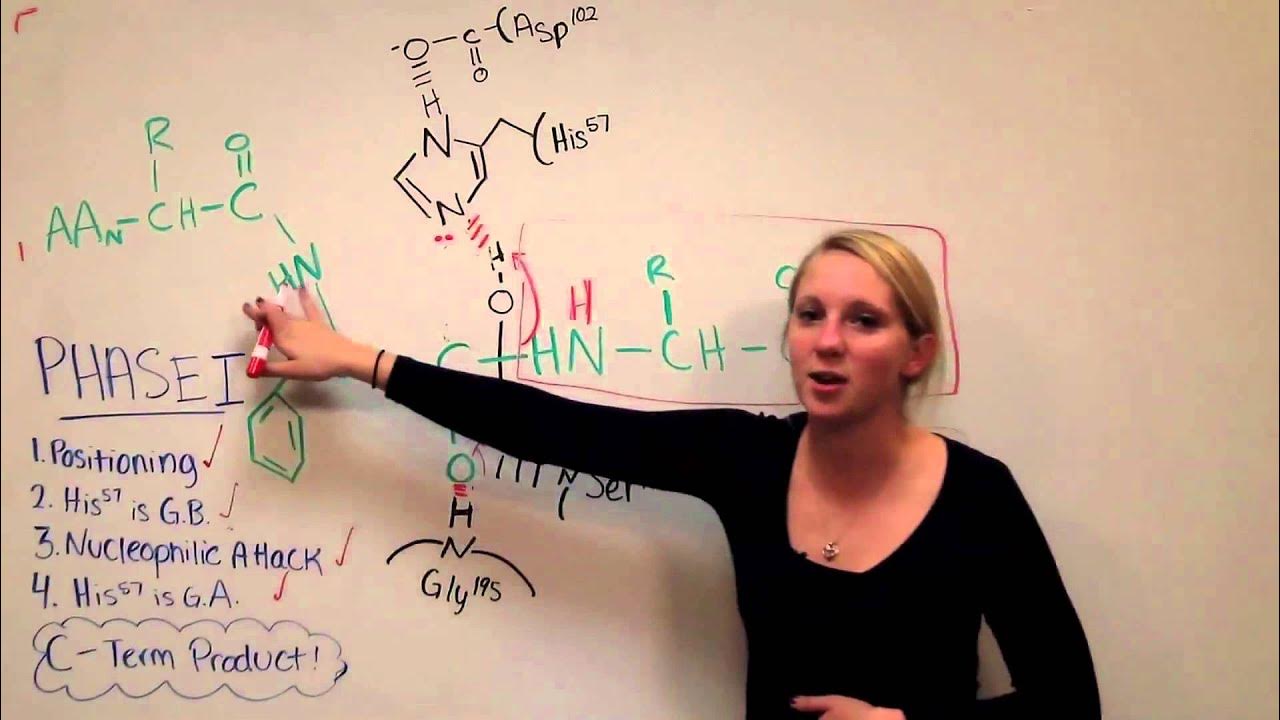
Chymotrypsin Mechanism
5.0 / 5 (0 votes)
