Outils 3c PART 4: La propagation des incertitudes
Summary
TLDRThis chemistry lecture focuses on the propagation of uncertainties in mathematical operations involving experimental measurements. It explains how to handle uncertainties when adding or subtracting measurements, emphasizing the need to add absolute uncertainties. The video uses a graduated cylinder example to illustrate the process of measuring volume changes with uncertainties. It also touches on the calculations required for multiplication and division, such as finding the density of ethanol by dividing mass by volume, and converting absolute uncertainties to relative uncertainties before combining them. The lecture concludes with a reminder about the importance of understanding precision and accuracy in measurements.
Takeaways
- 🔢 When adding or subtracting experimental measurements, you must also add the absolute uncertainties.
- 🧪 For volume measurements, use a graduated cylinder and consider the smallest division to estimate uncertainty.
- 📏 Uncertainty is represented by the half of the smallest division on the measuring instrument.
- ➕ When calculating the change in volume, subtract the initial volume from the final volume and consider the absolute uncertainties.
- 📉 The total uncertainty is the sum of the individual uncertainties from each measurement.
- 📊 For multiplication or division operations, combine the relative uncertainties.
- 📋 When finding the density of a substance like ethanol, measure both volume and mass, then divide the mass by volume.
- 🔍 The uncertainty of the balance and the graduated cylinder will differ based on the precision of the instruments used.
- 📐 Convert absolute uncertainties to relative uncertainties by dividing by the measured value and expressing it as a percentage.
- 🧮 To find the uncertainty in a calculated value like density, add the relative uncertainties together and then convert back to an absolute uncertainty.
- ⚖️ When reporting results, round the final uncertainty to a reasonable number of significant figures to reflect the precision of the measuring instruments.
Q & A
What is the main topic of the video script?
-The main topic of the video script is the propagation of uncertainties in mathematical operations within the context of chemistry, specifically focusing on the section about addition and subtraction of experimental measurements.
Why is it necessary to add absolute uncertainties when performing addition and subtraction of measurements?
-It is necessary to add absolute uncertainties when performing addition and subtraction to account for the potential errors in each individual measurement, ensuring that the combined result reflects the overall uncertainty of the operation.
What is the purpose of the graduated cylinder animation shown in the script?
-The purpose of the graduated cylinder animation is to illustrate how measurements are taken and how uncertainties are determined when using a graduated cylinder for volume measurements.
How is the uncertainty determined for a volume measurement using a graduated cylinder?
-The uncertainty for a volume measurement using a graduated cylinder is determined by taking the half of the smallest division on the cylinder, which in the script is half a milliliter (0.5 ml).
What is the process for calculating the change in volume when uncertainties are involved?
-The process involves measuring the initial volume, then the final volume after adding liquid, and subtracting the initial from the final to find the change. The uncertainty is then calculated by adding the absolute uncertainties of the initial and final measurements.
Why is it important to consider the instrument's precision when determining uncertainty?
-The precision of the instrument is crucial for determining uncertainty because it directly affects the smallest division that can be measured, which in turn influences the calculation of absolute uncertainty.
How does the script suggest handling uncertainties when performing multiplications or divisions?
-For multiplications or divisions, the script suggests adding the relative uncertainties together, which involves converting absolute uncertainties to relative uncertainties by dividing by the measured value and expressing them as percentages.
What is the example given in the script for calculating the density of ethanol?
-The script provides an example where the density of ethanol is calculated by dividing the mass of the liquid (measured in grams) by its volume (measured in milliliters), and then determining the uncertainty of this density by adding the relative uncertainties of the mass and volume measurements.
How are relative uncertainties converted back to absolute uncertainties in the context of the script?
-Relative uncertainties are converted back to absolute uncertainties by multiplying the relative uncertainty percentage by the calculated value, such as the density, to find the absolute uncertainty associated with that value.
What is the significance of rounding off the calculated uncertainty in the script?
-Rounding off the calculated uncertainty is significant because it ensures that the reported uncertainty is realistic and not overly precise, which would not reflect the true level of confidence in the measurement.
What is the difference between precision and accuracy mentioned in the script, and how does it relate to the propagation of uncertainties?
-Precision refers to the consistency of measurements, while accuracy is how close the measurements are to the true value. The script implies that understanding this difference is important for the propagation of uncertainties, as it affects how uncertainties are calculated and reported.
Outlines

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードMindmap

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードKeywords

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードHighlights

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードTranscripts

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレード関連動画をさらに表示
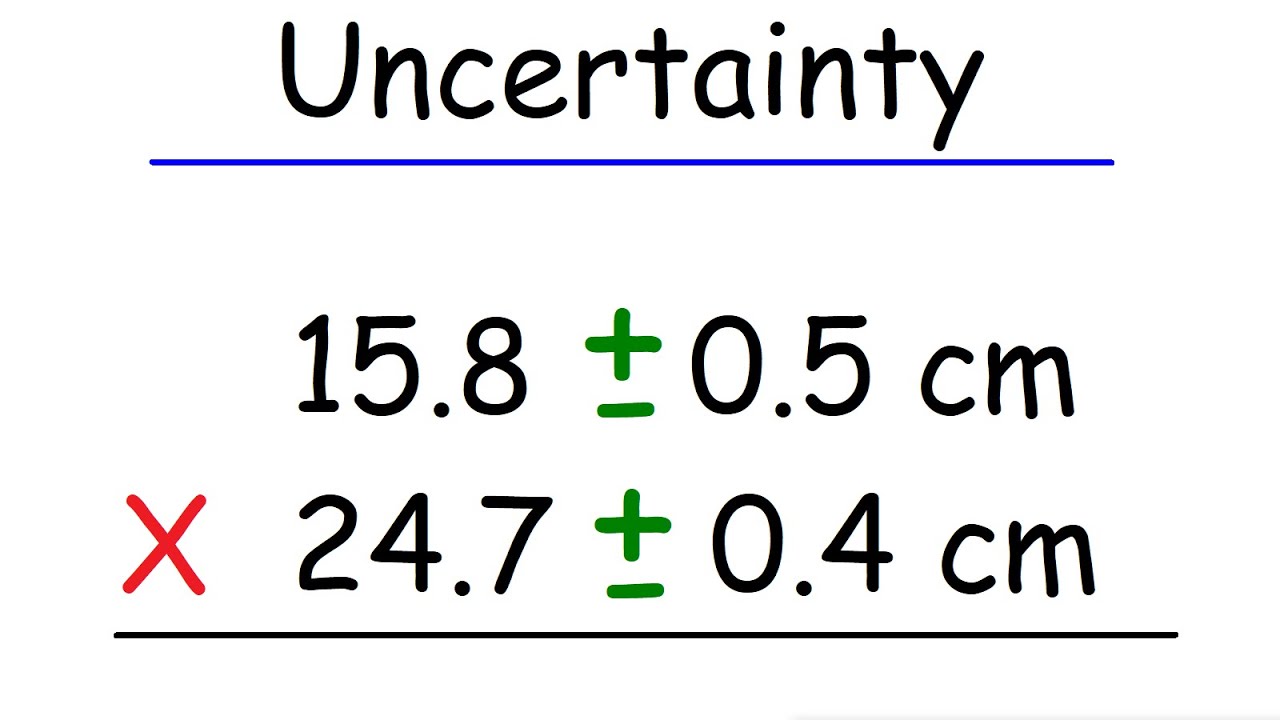
Uncertainty - Multiplication and Division

Propagação de incertezas - Física Experimental 1 - prof. Mariana UFU

TLE DRESSMAKING 7 - Lesson 6 APPLYING THE SYSTEM OF MEASUREMENT

Class 10 Science | Chemical Reactions And Equations | Characters of Chemical Reactions | Ashu Sir

BTEC Applied Science - Unit 5 Chemistry - Free radical substitution in Alkanes

1.2 Scientific Notation & Significant Figures | General Chemistry
5.0 / 5 (0 votes)
